Interim analysis of a phase 1 randomized clinical trial on the safety and immunogenicity of the mRNA-1283 SARS-CoV-2 vaccine in adults
- PMID: 37074202
- PMCID: PMC10128428
- DOI: 10.1080/21645515.2023.2190690
Interim analysis of a phase 1 randomized clinical trial on the safety and immunogenicity of the mRNA-1283 SARS-CoV-2 vaccine in adults
Abstract
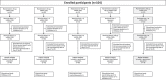
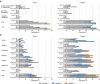
This interim analysis of an ongoing phase 1 randomized clinical trial evaluated the safety, reactogenicity, and immunogenicity of mRNA-1283, a next-generation SARS-CoV-2 messenger RNA (mRNA)-based vaccine encoding two segments of the spike protein (i.e. receptor binding and N-terminal domains). Healthy adults aged 18-55 years (n = 104) were randomized (1:1:1:1:1) to receive two doses of mRNA-1283 (10, 30, or 100 µg) or mRNA-1273 (100 µg) administered 28 days apart, or a single dose of mRNA-1283 (100 µg). Safety was assessed and immunogenicity was measured by serum neutralizing antibody (nAb) or binding antibody (bAb) responses. At the interim analysis, no safety concerns were identified and no serious adverse events, adverse events of special interest, or deaths were reported. Solicited systemic adverse reactions were more frequent with higher dose levels of mRNA-1283 than with mRNA-1273. At day 57, all dose levels of the 2-dose mRNA-1283 regimen (including the lowest dose level [10 µg]) induced robust nAb and bAb responses that were comparable to those of mRNA-1273 (100 µg). mRNA-1283 was generally safe in adults, with all dose levels of the 2-dose regimen (10, 30, and 100 µg) eliciting similar immunogenicity as the 2-dose mRNA-1273 regimen (100 µg).Clinical Trials Registration: Clinicaltrials.gov, NCT04813796.
Keywords: COVID-19; Severe acute respiratory syndrome coronavirus-2; clinical study; mRNA vaccines; vaccination.
Conflict of interest statement
PY and MH have no conflicts to declare. YDP, LS, AA, US, and RP are employees and shareholders in Moderna, Inc.
Figures


References
-
- FDA . Package Insert - SPIKEVAX. 2022. [accessed 2022 Mar 15]. https://www.fda.gov/media/155675/download.
-
- World Health Organization . Interim recommendations for use of the Moderna mRNA-1273 vaccine against COVID-19. 2022. [accessed 2023 Mar 13]. https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE-reco....
-
- Bruxvoort KJ, Sy LS, Qian L, Ackerson BK, Luo Y, Lee GS, Tian Y, Florea A, Takhar HS, Tubert JE, et al. Real-world effectiveness of the mRNA-1273 vaccine against COVID-19: interim results from a prospective observational cohort study. Lancet Reg Health Am. 2021:100134. doi: 10.2139/ssrn.3916094. - DOI - PMC - PubMed
-
- Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, Tang P, Hasan MR, Malek JA, Coyle P, Ayoub HH, Al Kanaani Z, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021;27(9):1614–21. doi: 10.1038/s41591-021-01446-y. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
