Intestinal vitamin D receptor protects against extraintestinal breast cancer tumorigenesis
- PMID: 37074210
- PMCID: PMC10120454
- DOI: 10.1080/19490976.2023.2202593
Intestinal vitamin D receptor protects against extraintestinal breast cancer tumorigenesis
Abstract
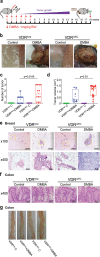
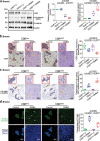
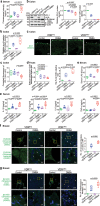
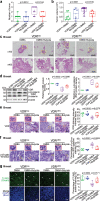
The microbiota plays critical roles in regulating the function and health of the intestine and extraintestinal organs. A fundamental question is whether an intestinal-microbiome-breast axis exists during the development of breast cancer. If so, what are the roles of host factors? Vitamin D receptor (VDR) involves host factors and the human microbiome. Vdr gene variation shapes the human microbiome, and VDR deficiency leads to dysbiosis. We hypothesized that intestinal VDR protects hosts against tumorigenesis in the breast. We examined a 7,12-dimethylbenzanthracene (DMBA)-induced breast cancer model in intestinal epithelial VDR knockout (VDRΔIEC) mice with dysbiosis. We reported that VDRΔIEC mice with dysbiosis are more susceptible to breast cancer induced by DMBA. Intestinal and breast microbiota analysis showed that VDR deficiency leads to a bacterial profile shift from normal to susceptible to carcinogenesis. We found enhanced bacterial staining within breast tumors. At the molecular and cellular levels, we identified the mechanisms by which intestinal epithelial VDR deficiency led to increased gut permeability, disrupted tight junctions, microbial translocation, and enhanced inflammation, thus increasing tumor size and number in the breast. Furthermore, treatment with the beneficial bacterial metabolite butyrate or the probiotic Lactobacillus plantarum reduced breast tumors, enhanced tight junctions, inhibited inflammation, increased butyryl-CoA transferase, and decreased levels of breast Streptococcus bacteria in VDRΔIEC mice. The gut microbiome contributes to the pathogenesis of diseases not only in the intestine but also in the breast. Our study provides insights into the mechanism by which intestinal VDR dysfunction and gut dysbiosis lead to a high risk of extraintestinal tumorigenesis. Gut-tumor-microbiome interactions represent a new target in the prevention and treatment of breast cancer.
Keywords: Dysbiosis; Lactobacillus plantarum; VDR; barrier function; breast cancer; butyrate; butyrate-producing bacteria; gut-breast-axis; inflammation; probiotics; tight junctions.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



Similar articles
-
Vitamin D Receptor Protects Against Dysbiosis and Tumorigenesis via the JAK/STAT Pathway in Intestine.Cell Mol Gastroenterol Hepatol. 2020;10(4):729-746. doi: 10.1016/j.jcmgh.2020.05.010. Epub 2020 Jun 1. Cell Mol Gastroenterol Hepatol. 2020. PMID: 32497792 Free PMC article.
-
Bacterial translocation and barrier dysfunction enhance colonic tumorigenesis.Neoplasia. 2023 Jan;35:100847. doi: 10.1016/j.neo.2022.100847. Epub 2022 Nov 2. Neoplasia. 2023. PMID: 36334333 Free PMC article.
-
Lack of Vitamin D Receptor Causes Dysbiosis and Changes the Functions of the Murine Intestinal Microbiome.Clin Ther. 2015 May 1;37(5):996-1009.e7. doi: 10.1016/j.clinthera.2015.04.004. Clin Ther. 2015. PMID: 26046242
-
Vitamin D Modulates Intestinal Microbiota in Inflammatory Bowel Diseases.Int J Mol Sci. 2020 Dec 31;22(1):362. doi: 10.3390/ijms22010362. Int J Mol Sci. 2020. PMID: 33396382 Free PMC article. Review.
-
Breast and gut microbiome in health and cancer.Genes Dis. 2020 Aug 20;8(5):581-589. doi: 10.1016/j.gendis.2020.08.002. eCollection 2021 Sep. Genes Dis. 2020. PMID: 34291130 Free PMC article. Review.
Cited by
-
Current and future research on the association between gut microbiota and breast cancer.Front Microbiol. 2023 Oct 30;14:1272275. doi: 10.3389/fmicb.2023.1272275. eCollection 2023. Front Microbiol. 2023. PMID: 38029117 Free PMC article. Review.
-
Role of gut microbiome in suppression of cancers.Gut Microbes. 2025 Dec;17(1):2495183. doi: 10.1080/19490976.2025.2495183. Epub 2025 Apr 20. Gut Microbes. 2025. PMID: 40254597 Free PMC article. Review.
-
Poking at probiotic mechanisms and microbial implications in cancer prevention and treatment.Gut. 2024 Aug 8;73(9):1408-1409. doi: 10.1136/gutjnl-2024-332560. Gut. 2024. PMID: 38889962 Free PMC article. No abstract available.
-
The microbiota in breast cancer: dysbiosis, microbial metabolites, and therapeutic implications.Am J Cancer Res. 2025 Apr 15;15(4):1384-1409. doi: 10.62347/ZJCF2843. eCollection 2025. Am J Cancer Res. 2025. PMID: 40371158 Free PMC article. Review.
-
Synergistic Effects of Riluzole and Sodium Butyrate on Barrier Function and Disease Progression of Amyotrophic Lateral Sclerosis Through the Gut-Neuron Axis.Compr Physiol. 2025 Apr;15(2):e70009. doi: 10.1002/cph4.70009. Compr Physiol. 2025. PMID: 40176466 Free PMC article.
References
-
- Abreu MT, Kantorovich V, Vasiliauskas EA, Gruntmanis U, Matuk R, Daigle K, Chen S, Zehnder D, Lin YC, Yang H, et al. Measurement of vitamin D levels in inflammatory bowel disease patients reveals a subset of Crohn’s disease patients with elevated 1,25-dihydroxy vitamin D and low bone mineral density. Gut. 2004;53(8):1129–1136. doi:10.1136/gut.2003.036657. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
