Metabolic Consequences of Polyphosphate Synthesis and Imminent Phosphate Limitation
- PMID: 37074217
- PMCID: PMC10294617
- DOI: 10.1128/mbio.00102-23
Metabolic Consequences of Polyphosphate Synthesis and Imminent Phosphate Limitation
Abstract
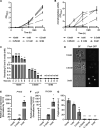
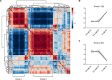
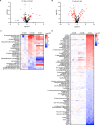
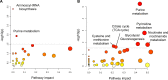
Cells stabilize intracellular inorganic phosphate (Pi) to compromise between large biosynthetic needs and detrimental bioenergetic effects of Pi. Pi homeostasis in eukaryotes uses Syg1/Pho81/Xpr1 (SPX) domains, which are receptors for inositol pyrophosphates. We explored how polymerization and storage of Pi in acidocalcisome-like vacuoles supports Saccharomyces cerevisiae metabolism and how these cells recognize Pi scarcity. Whereas Pi starvation affects numerous metabolic pathways, beginning Pi scarcity affects few metabolites. These include inositol pyrophosphates and ATP, a low-affinity substrate for inositol pyrophosphate-synthesizing kinases. Declining ATP and inositol pyrophosphates may thus be indicators of impending Pi limitation. Actual Pi starvation triggers accumulation of the purine synthesis intermediate 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), which activates Pi-dependent transcription factors. Cells lacking inorganic polyphosphate show Pi starvation features already under Pi-replete conditions, suggesting that vacuolar polyphosphate supplies Pi for metabolism even when Pi is abundant. However, polyphosphate deficiency also generates unique metabolic changes that are not observed in starving wild-type cells. Polyphosphate in acidocalcisome-like vacuoles may hence be more than a global phosphate reserve and channel Pi to preferred cellular processes. IMPORTANCE Cells must strike a delicate balance between the high demand of inorganic phosphate (Pi) for synthesizing nucleic acids and phospholipids and its detrimental bioenergetic effects by reducing the free energy of nucleotide hydrolysis. The latter may stall metabolism. Therefore, microorganisms manage the import and export of phosphate, its conversion into osmotically inactive inorganic polyphosphates, and their storage in dedicated organelles (acidocalcisomes). Here, we provide novel insights into metabolic changes that yeast cells may use to signal declining phosphate availability in the cytosol and differentiate it from actual phosphate starvation. We also analyze the role of acidocalcisome-like organelles in phosphate homeostasis. This study uncovers an unexpected role of the polyphosphate pool in these organelles under phosphate-rich conditions, indicating that its metabolic roles go beyond that of a phosphate reserve for surviving starvation.
Keywords: SPX domains; Saccharomyces cerevisiae; acidocalcisome; phosphate signalling; polyphosphate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

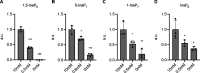
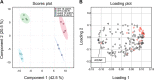

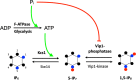
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
