The Impact of Mmu17 Non-Hsa21 Orthologous Genes in the Ts65Dn Mouse Model of Down Syndrome: The Gold Standard Refuted
- PMID: 37074246
- PMCID: PMC10330375
- DOI: 10.1016/j.biopsych.2023.02.012
The Impact of Mmu17 Non-Hsa21 Orthologous Genes in the Ts65Dn Mouse Model of Down Syndrome: The Gold Standard Refuted
Abstract
Background: Despite successful preclinical treatment studies to improve neurocognition in the Ts65Dn mouse model of Down syndrome, translation to humans has failed. This raises questions about the appropriateness of the Ts65Dn mouse as the gold standard. We used the novel Ts66Yah mouse that carries an extra chromosome and the identical segmental Mmu16 trisomy as Ts65Dn without the Mmu17 non-Hsa21 orthologous region.
Methods: Forebrains from embryonic day 18.5 Ts66Yah and Ts65Dn mice, along with euploid littermate controls, were used for gene expression and pathway analyses. Behavioral experiments were performed in neonatal and adult mice. Because male Ts66Yah mice are fertile, parent-of-origin transmission of the extra chromosome was studied.
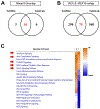
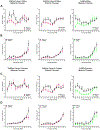
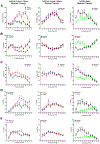
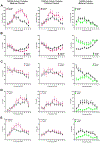
Results: Forty-five protein-coding genes mapped to the Ts65Dn Mmu17 non-Hsa21 orthologous region; 71%-82% are expressed during forebrain development. Several of these genes are uniquely overexpressed in Ts65Dn embryonic forebrain, producing major differences in dysregulated genes and pathways. Despite these differences, the primary Mmu16 trisomic effects were highly conserved in both models, resulting in commonly dysregulated disomic genes and pathways. Delays in motor development, communication, and olfactory spatial memory were present in Ts66Yah but more pronounced in Ts65Dn neonates. Adult Ts66Yah mice showed milder working memory deficits and sex-specific effects in exploratory behavior and spatial hippocampal memory, while long-term memory was preserved.
Conclusions: Our findings suggest that triplication of the non-Hsa21 orthologous Mmu17 genes significantly contributes to the phenotype of the Ts65Dn mouse and may explain why preclinical trials that used this model have unsuccessfully translated to human therapies.
Keywords: Down syndrome; Mmu17 non-Hsa21 orthologous genes; Mouse models; Phenotype; Trisomy 21; Ts65Dn; Ts66Yah.
Published by Elsevier Inc.
Figures



Similar articles
-
Human chromosome 21 orthologous region on mouse chromosome 17 is a major determinant of Down syndrome-related developmental cognitive deficits.Hum Mol Genet. 2014 Feb 1;23(3):578-89. doi: 10.1093/hmg/ddt446. Epub 2013 Sep 16. Hum Mol Genet. 2014. PMID: 24041763 Free PMC article.
-
Mouse models of Down syndrome: gene content and consequences.Mamm Genome. 2016 Dec;27(11-12):538-555. doi: 10.1007/s00335-016-9661-8. Epub 2016 Aug 18. Mamm Genome. 2016. PMID: 27538963 Free PMC article. Review.
-
Dendritic phenotype and proliferation potency in the hippocampal dentate gyrus of the Ts66Yah model of Down syndrome.Neurosci Lett. 2025 Feb 28;850:138156. doi: 10.1016/j.neulet.2025.138156. Epub 2025 Feb 8. Neurosci Lett. 2025. PMID: 39929391
-
Sexually dimorphic DYRK1A overexpression on postnatal day 15 in the Ts65Dn mouse model of Down syndrome: Effects of pharmacological targeting on behavioral phenotypes.Pharmacol Biochem Behav. 2022 Jun;217:173404. doi: 10.1016/j.pbb.2022.173404. Epub 2022 May 14. Pharmacol Biochem Behav. 2022. PMID: 35576991 Free PMC article.
-
Mouse models of cognitive disorders in trisomy 21: a review.Behav Genet. 2006 May;36(3):387-404. doi: 10.1007/s10519-006-9056-9. Epub 2006 Mar 8. Behav Genet. 2006. PMID: 16523244 Review.
Cited by
-
Neocortical neuronal production and maturation defects in the TcMAC21 mouse model of Down syndrome.iScience. 2023 Nov 2;26(12):108379. doi: 10.1016/j.isci.2023.108379. eCollection 2023 Dec 15. iScience. 2023. PMID: 38025769 Free PMC article.
-
Overexpression screen of chromosome 21 genes reveals modulators of Sonic hedgehog signaling relevant to Down syndrome.Dis Model Mech. 2023 Apr 1;16(4):dmm049712. doi: 10.1242/dmm.049712. Epub 2023 Apr 13. Dis Model Mech. 2023. PMID: 36995257 Free PMC article.
-
Exploring crystallized and fluid intelligence in down syndrome using graph theory.Sci Rep. 2024 Oct 10;14(1):23738. doi: 10.1038/s41598-024-74815-5. Sci Rep. 2024. PMID: 39390071 Free PMC article.
-
Glutamatergic synaptic deficits in the prefrontal cortex of the Ts65Dn mouse model for Down syndrome.Front Neurosci. 2023 Sep 28;17:1171797. doi: 10.3389/fnins.2023.1171797. eCollection 2023. Front Neurosci. 2023. PMID: 37841687 Free PMC article.
-
Neuronal Excitability in Trisomy 21-Up or Down?Epilepsy Curr. 2024 Oct 16;24(6):440-442. doi: 10.1177/15357597241285779. eCollection 2024 Nov-Dec. Epilepsy Curr. 2024. PMID: 39540135 Free PMC article. No abstract available.
References
-
- Davisson MT, Schmidt C, Akeson EC (1990): Segmental trisomy of murine chromosome 16: A new model system for studying Down syndrome. Prog Clin Biol Res 360:263–280. - PubMed
-
- Davisson MT, Schmidt C, Reeves RH, Irving NG, Akeson EC, Harris BS, Bronson RT (1993): Segmental trisomy as a mouse model for Down syndrome. Prog Clin Biol Res 384:117–133. - PubMed
-
- Muñiz Moreno MDM, Brault V, Birling MC, Pavlovic G, Herault Y (2020): Modeling Down syndrome in animals from the early stage to the 4.0 models and next. Prog Brain Res 251:91–143. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

