Evaluation of the diagnostic efficacy of EC-Test for latent tuberculosis infection in ambulatory people with HIV
- PMID: 37074384
- PMCID: PMC10481920
- DOI: 10.1097/QAD.0000000000003573
Evaluation of the diagnostic efficacy of EC-Test for latent tuberculosis infection in ambulatory people with HIV
Abstract
Background: Latent tuberculosis infection (LTBI) co-infected with human immunodeficiency virus (HIV) is more likely to develop into active tuberculosis (ATB), recombinant Mycobacterium tuberculosis fusion protein ESAT6/CFP10 (EC-Test) is a latest developed method for LTBI. Compared with the interferon γ release test assays (IGRAs), the diagnostic performance of EC-Test to LTBI screening in HIV needs to be evaluated.
Methods: A population-based multicenter prospective study was conducted in Guangxi Province, China. The baseline data was collected and LTBI were measured by QuantiFERON-TB Gold In-Tube (QFT-GIT), EC-Test and T-cell spot of the TB assay (T-SPOT.TB).
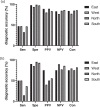
Results: A total of 1478 patients were enrolled. when taking T-SPOT.TB as reference, the value of sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and consistency that EC-Test to diagnosis LTBI in HIV was 40.42, 97.98, 85.26, 85.04 and 85.06% respectively; when taking QFT-GIT as reference, the value was 36.00, 92.57, 55.10, 85.09 and 81.13%, respectively. When the CD4 + cell count was <200 cells/μl, the accuracies of EC-Test to T-SPOT.TB and QFT-GIT were 87.12 and 88.89%, respectively; when it was 200 ≤ CD4 + ≤ 500 cells/μl, the accuracies of EC-Test was 86.20 and 83.18%, respectively; when the CD4 + cell count >500 cells/μl, the accuracies of EC-Test were 84.29 and 77.94%, respectively. The incidence of adverse reactions in EC-Test was 34.23% and the serious adverse reactions were 1.15%.
Conclusion: EC-Test has good consistency compared with IGRAs in detecting LTBI in HIV no matter in different immunosuppression status or different regions, and the safety of EC-Test is also well, suitable for LTBI screening in HIV in high prevalence settings.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
There are no conflicts of interest.
Figures
Similar articles
-
Prevalence of latent tuberculosis infection in persons with and without human immunodeficiency virus infection using two interferon-gamma release assays and tuberculin skin test in a low human immunodeficiency virus prevalence, intermediate tuberculosis-burden country.J Microbiol Immunol Infect. 2016 Oct;49(5):729-736. doi: 10.1016/j.jmii.2014.08.010. Epub 2014 Nov 1. J Microbiol Immunol Infect. 2016. PMID: 25442858
-
Interferon gamma release assays for Diagnostic Evaluation of Active tuberculosis (IDEA): test accuracy study and economic evaluation.Health Technol Assess. 2019 May;23(23):1-152. doi: 10.3310/hta23230. Health Technol Assess. 2019. PMID: 31138395 Free PMC article.
-
Optimal Testing Choice and Diagnostic Strategies for Latent Tuberculosis Infection Among US-Born People Living with Human Immunodeficiency Virus (HIV).Clin Infect Dis. 2021 Oct 5;73(7):e2278-e2284. doi: 10.1093/cid/ciaa1135. Clin Infect Dis. 2021. PMID: 32761083 Free PMC article.
-
[Evolution of IGRA researches].Kekkaku. 2008 Sep;83(9):641-52. Kekkaku. 2008. PMID: 18979999 Review. Japanese.
-
Fourth-Generation QuantiFERON-TB Gold Plus: What Is the Evidence?J Clin Microbiol. 2020 Aug 24;58(9):e01950-19. doi: 10.1128/JCM.01950-19. Print 2020 Aug 24. J Clin Microbiol. 2020. PMID: 32493779 Free PMC article. Review.
Cited by
-
Effect of CD4 count on Mycobacterium tuberculosis infection rates in people living with HIV: a comparative study in prison and community.Sci Rep. 2024 Nov 2;14(1):26386. doi: 10.1038/s41598-024-77250-8. Sci Rep. 2024. PMID: 39488608 Free PMC article.
-
Single-Cell Transcriptomics of Mtb/HIV Co-Infection.Cells. 2023 Sep 17;12(18):2295. doi: 10.3390/cells12182295. Cells. 2023. PMID: 37759517 Free PMC article. Review.
References
-
- World Health Organization (WHO). Global tuberculosis report 2020. WHO: Geneva; 2021.
-
- Cui X, Gao L. Management of latent tuberculosis infection in China: exploring solutions suitable for high-burden countries. Int J Infect Dis 2020; 92S:S37–S40. - PubMed
-
- Pai M, Behr M. Latent Mycobacterium tuberculosis infection and interferon-gamma release assays. Microbiol Spectr 2016; 4: doi: 10.1128/microbiolspec. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials