Myeloid Src-family kinases are critical for neutrophil-mediated autoinflammation in gout and motheaten models
- PMID: 37074415
- PMCID: PMC10120404
- DOI: 10.1084/jem.20221010
Myeloid Src-family kinases are critical for neutrophil-mediated autoinflammation in gout and motheaten models
Abstract
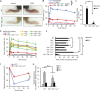
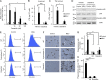
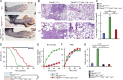
Autoinflammatory diseases include a number of monogenic systemic inflammatory diseases, as well as acquired autoinflammatory diseases such as gout. Here, we show that the myeloid Src-family kinases Hck, Fgr, and Lyn are critical for experimental models of gout, as well as for genetically determined systemic inflammation in the Ptpn6me-v/me-v (motheaten viable) mouse model. The Hck-/-Fgr-/-Lyn-/- mutation abrogated various monosodium urate (MSU) crystal-induced pro-inflammatory responses of neutrophils, and protected mice from the development of gouty arthritis. The Src-family inhibitor dasatinib abrogated MSU crystal-induced responses of human neutrophils and reduced experimental gouty arthritis in mice. The Hck-/-Fgr-/-Lyn-/- mutation also abrogated spontaneous inflammation and prolonged the survival of the Ptpn6me-v/me-v mice. Spontaneous adhesion and superoxide release of Ptpn6me-v/me-v neutrophils were also abolished by the Hck-/-Fgr-/-Lyn-/- mutation. Excessive activation of tyrosine phosphorylation pathways in myeloid cells may characterize a subset of autoinflammatory diseases.
© 2023 Futosi et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures








References
-
- Abe, K., Cox A., Takamatsu N., Velez G., Laxer R.M., Tse S.M.L., Mahajan V.B., Bassuk A.G., Fuchs H., Ferguson P.J., and Hrabe de Angelis M.. 2019. Gain-of-function mutations in a member of the Src family kinases cause autoinflammatory bone disease in mice and humans. Proc. Natl. Acad. Sci. USA. 116:11872–11877. 10.1073/pnas.1819825116 - DOI - PMC - PubMed
-
- Abe, K., Fuchs H., Boersma A., Hans W., Yu P., Kalaydjiev S., Klaften M., Adler T., Calzada-Wack J., Mossbrugger I., et al. . 2011. A novel N-ethyl-N-nitrosourea-induced mutation in phospholipase Cγ2 causes inflammatory arthritis, metabolic defects, and male infertility in vitro in a murine model. Arthritis Rheum. 63:1301–1311. 10.1002/art.30280 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

