Shigella in Africa: New Insights From the Vaccine Impact on Diarrhea in Africa (VIDA) Study
- PMID: 37074444
- PMCID: PMC10116563
- DOI: 10.1093/cid/ciac969
Shigella in Africa: New Insights From the Vaccine Impact on Diarrhea in Africa (VIDA) Study
Abstract
Background: We evaluated the burden of Shigella spp from children aged 0-59 months with medically attended moderate-to-severe diarrhea and matched controls at sites in Mali, The Gambia, and Kenya participating in the Vaccine Impact on Diarrhea in Africa (VIDA) study from 2015 to 2018.
Methods: Shigella spp were identified using coprocultures and serotyping in addition to quantitative polymerase chain reaction (qPCR). Episode-specific attributable fractions (AFe) for Shigella were calculated using Shigella DNA quantity; cases with AFe ≥0.5 were considered to have shigellosis.
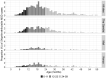
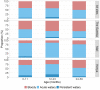
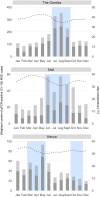
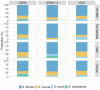
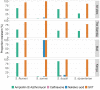
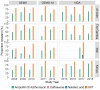
Results: The prevalence of Shigella was determined to be 359 of 4840 (7.4%) cases and 83 of 6213 (1.3%) controls by culture, and 1641 of 4836 (33.9%) cases and 1084 of 4846 (22.4%) controls by qPCR (cycle threshold <35); shigellosis was higher in The Gambia (30.8%) than in Mali (9.3%) and Kenya (18.7%). Bloody diarrhea attributed to Shigella was more common in 24- to 59-month-old children (50.1%) than 0- to 11-month-old infants (39.5%). The Shigella flexneri serogroup predominated among cases (67.6% of isolates), followed by Shigella sonnei (18.2%), Shigella boydii (11.8%), and Shigella dysenteriae (2.3%). The most frequent S. flexneri serotypes were 2a (40.6%), 1b (18.8%), 6 (17.5%), 3a (9.0%), and 4a (5.1%). Drug-specific resistance among 353 (98.3%) Shigella cases with AMR data was as follows: trimethoprim-sulfamethoxazole (94.9%), ampicillin (48.4%), nalidixic acid (1.7%), ceftriaxone (0.3%), azithromycin (0.3%), and ciprofloxacin (0.0%).
Conclusions: A high prevalence of shigellosis continues in sub-Saharan Africa. Strains are highly resistant to commonly used antibiotics while remaining susceptible to ciprofloxacin, ceftriaxone, and azithromycin.
Keywords: Africa; Shigella; children; diarrhea; dysentery.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. K. L. K. reports consultation fees and travel support from PATH and the University of Washington related to diarrheal diseases and grant support to her institution from the National Institutes of Health, Institut Pasteur, and the Bill & Melinda Gates Foundation. M.-A. W. reports receiving funding from the CDC and the Institute of Tropical Medicine. S. M. T. reports multiple grants (paid to institution) from the National Institutes of Health, Bill & Melinda Gates Foundation, Wellcome Trust, Affinivax, Lumen Biosciences, PATH, and Medical Research Council; payments as royalties related to intellectual property for Salmonella vaccines and Klebsiella/Pseudomonas vaccines; consulting fees and travel support from the University of Washington for a grant proposal; multiple planned, issued, and pending patents on Salmonella, Klebsiella, and Pseudomonas vaccines; and multiple unpaid committee roles with the American Society of Tropical Medicine and Hygiene. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

