Consistent Effects of Early Remdesivir on Symptoms and Disease Progression Across At-Risk Outpatient Subgroups: Treatment Effect Heterogeneity in PINETREE Study
- PMID: 37074613
- PMCID: PMC10113728
- DOI: 10.1007/s40121-023-00789-y
Consistent Effects of Early Remdesivir on Symptoms and Disease Progression Across At-Risk Outpatient Subgroups: Treatment Effect Heterogeneity in PINETREE Study
Abstract
Introduction: In the PINETREE study, early remdesivir treatment reduced risk of coronavirus disease 2019 (COVID-19)-related hospitalizations or all-cause death versus placebo by 87% by day 28 in high-risk, non-hospitalized patients. Here we report results of assessment of heterogeneity of treatment effect (HTE) of early outpatient remdesivir, focusing on time from symptom onset and number of baseline risk factors (RFs).
Methods: PINETREE was a double-blind, placebo-controlled trial of non-hospitalized patients with COVID-19 who were randomized within 7 days of symptom onset and had ≥ 1 RF for disease progression (age ≥ 60 years, obesity [body mass index ≥ 30], or certain coexisting medical conditions). Patients received remdesivir intravenously (200 mg on day 1 and 100 mg on days 2 and 3) or placebo.
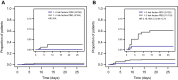
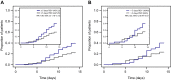
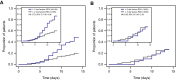
Results: In this subgroup analysis, HTE of remdesivir by time from symptom onset at treatment initiation and number of baseline RFs was not detected. Treatment with remdesivir reduced COVID-19-related hospitalizations independent of stratification by time from symptom onset to randomization. Of patients enrolled ≤ 5 days from symptom onset, 1/201 (0.5%) receiving remdesivir and 9/194 (4.6%) receiving placebo were hospitalized (hazard ratio [HR] 0.10; 95% confidence interval [CI] 0.01-0.82). Of those enrolled at > 5 days from symptom onset, 1/78 (1.3%) receiving remdesivir and 6/89 (6.7%) receiving placebo were hospitalized (HR 0.19; 95% CI 0.02-1.61). Remdesivir was also effective in reducing COVID-19-related hospitalizations when stratified by number of baseline RFs for severe disease. Of patients with ≤ 2 RFs, 0/159 (0.0%) receiving remdesivir and 4/164 (2.4%) receiving placebo were hospitalized; of those with ≥ 3 RFs, 2/120 (1.7%) receiving remdesivir and 11/119 (9.2%) receiving placebo were hospitalized (HR 0.16; 95% CI 0.04-0.73).
Conclusions: In the outpatient setting, benefit of remdesivir initiated within 7 days of symptoms appeared to be consistent across patients with RFs. Therefore, it may be reasonable to broadly treat patients with remdesivir regardless of comorbidities.
Trial registration: ClinicalTrials.gov number NCT04501952.
Keywords: Antiviral; COVID-19; Coronavirus; Outpatients; Remdesivir; SARS-CoV-2.
© 2023. The Author(s).
Conflict of interest statement
Monica Ramchandani, Yuan Tian, Emon Elboudwarej, Shuguang Chen, Jairo M. Montezuma-Rusca, Anu Osinusi, Lauren Rodriguez, and Charlotte Hedskog are employees and stockholders of Gilead Sciences. Samuel M. Brown received funding for COVID-19 research from NIH, Department of Defense (DoD), and CDC outside the present study and chairs a Data and Safety Monitoring Board for Hamilton Ventilators, outside the present study. Morgan J. Katz received research funding from CDC and the Agency for Healthcare and Research Quality and consulting fees from Artis Health Systems and Skinflique outside the present study. Adit A. Ginde received funding for COVID-19 research from NIH, DoD, CDC, Faron Pharmaceuticals, and AbbVie outside the present study. Kavita Juneja was an employee of Gilead Sciences at the time this work was done and is now an employee of Corcept Therapeutics. Joshua T. Schiffer was an investigator for the PINETREE study; he assisted Gilead in designing the clinical trial but was not compensated for this work. Dr. Schiffer declares no additional personal interests. Carlos Vaca declares no personal interests. Robert L. Gottlieb has been a consultant for AbbVie, Gilead Sciences, Johnson & Johnson, Roivant Pharmaceuticals, Roche Pharmaceuticals, GSK, and Eli Lilly. Dr. Gottlieb is also a national coordinating principal investigator for Johnson & Johnson, served on an academic steering committee for Roivant Pharmaceuticals, and received a gift-in-kind to Baylor Scott and White Research Institute to facilitate NCT03383419 from Gilead Sciences. Dr. Gottlieb owns de minimis stock in AbCellera Biologics and has served as a speaker for Pfizer outside of the scope of COVID-19. Joshua A. Hill received research funding from Gilead Sciences related to the current work; research funding from Takeda, Allovir, Karius, Merck, and Deverra outside the current work; and consulting fees from Amplyx, Allovir, Allogene, Takeda, and CRISPR outside the current work. Richard Gilson was an investigator for the PINETREE study; he has no other interests to declare. Roger Paredes received funding for COVID-19 research from NIH, Gilead, Lilly, and PharmaMar outside the present study, and has participated in COVID-19-related advisory boards for Gilead, MSD, Lilly, Roche, Atea, GSK, and Pfizer.
Figures

Similar articles
-
Remdesivir: A Review in COVID-19.Drugs. 2023 Sep;83(13):1215-1237. doi: 10.1007/s40265-023-01926-0. Epub 2023 Aug 17. Drugs. 2023. PMID: 37589788 Free PMC article. Review.
-
Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients.N Engl J Med. 2022 Jan 27;386(4):305-315. doi: 10.1056/NEJMoa2116846. Epub 2021 Dec 22. N Engl J Med. 2022. PMID: 34937145 Free PMC article. Clinical Trial.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Reconvalescent plasma/camostat mesylate in early SARS-CoV-2 Q-PCR positive high-risk individuals (RES-Q-HR): a structured summary of a study protocol for a randomized controlled trial.Trials. 2021 May 17;22(1):343. doi: 10.1186/s13063-021-05181-0. Trials. 2021. PMID: 34001215 Free PMC article.
-
Remdesivir in Treatment of COVID-19: A Systematic Benefit-Risk Assessment.Drug Saf. 2020 Jul;43(7):645-656. doi: 10.1007/s40264-020-00952-1. Drug Saf. 2020. PMID: 32468196 Free PMC article.
Cited by
-
No Remdesivir Resistance Observed in the Phase 3 Severe and Moderate COVID-19 SIMPLE Trials.Viruses. 2024 Mar 31;16(4):546. doi: 10.3390/v16040546. Viruses. 2024. PMID: 38675889 Free PMC article. Clinical Trial.
-
Remdesivir: A Review in COVID-19.Drugs. 2023 Sep;83(13):1215-1237. doi: 10.1007/s40265-023-01926-0. Epub 2023 Aug 17. Drugs. 2023. PMID: 37589788 Free PMC article. Review.
-
Development of a metabolome-based respiratory infection prognostic during COVID-19 arrival.mBio. 2025 Jan 8;16(1):e0334323. doi: 10.1128/mbio.03343-23. Epub 2024 Nov 22. mBio. 2025. PMID: 39576111 Free PMC article.
-
Remdesivir is Associated with Reduced Mortality in Patients Hospitalized for COVID-19 Not Requiring Supplemental Oxygen.Open Forum Infect Dis. 2024 Apr 16;11(6):ofae202. doi: 10.1093/ofid/ofae202. eCollection 2024 Jun. Open Forum Infect Dis. 2024. PMID: 38894848 Free PMC article.
-
BioMapAI: Artificial Intelligence Multi-Omics Modeling of Myalgic Encephalomyelitis / Chronic Fatigue Syndrome.bioRxiv [Preprint]. 2025 Feb 13:2024.06.24.600378. doi: 10.1101/2024.06.24.600378. bioRxiv. 2025. PMID: 38979186 Free PMC article. Preprint.
References
-
- Muthuri SG, Venkatesan S, Myles PR, et al. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med. 2014;2:395–404. doi: 10.1016/S2213-2600(14)70041-4. - DOI - PMC - PubMed
-
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/. Accessed 1 June 2021.
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

