Mice Deficient in TAZ (Wwtr1) Demonstrate Clinical Features of Late-Onset Fuchs' Endothelial Corneal Dystrophy
- PMID: 37074694
- PMCID: PMC10132321
- DOI: 10.1167/iovs.64.4.22
Mice Deficient in TAZ (Wwtr1) Demonstrate Clinical Features of Late-Onset Fuchs' Endothelial Corneal Dystrophy
Abstract
Purpose: We sought to define the role of Wwtr1 in murine ocular structure and function and determine the role of mechanotransduction in Fuchs' endothelial corneal dystrophy (FECD), with emphasis on interactions between corneal endothelial cells (CEnCs) and Descemet's membrane (DM).
Methods: A Wwtr1 deficient mouse colony was established, and advanced ocular imaging, atomic force microscope (AFM), and histology/immunofluorescence were performed. Corneal endothelial wound healing was assessed using cryoinjury and phototherapeutic keratectomy in Wwtr1 deficient mice. Expression of WWTR1/TAZ was determined in the corneal endothelium from normal and FECD-affected patients; WWTR1 was screened for coding sequence variants in this FECD cohort.
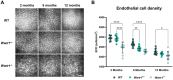
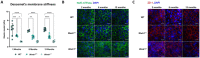
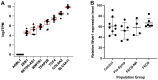
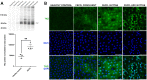
Results: Mice deficient in Wwtr1 had reduced CEnC density, abnormal CEnC morphology, softer DM, and thinner corneas versus wildtype controls by 2 months of age. Additionally, CEnCs had altered expression and localization of Na/K-ATPase and ZO-1. Further, Wwtr1 deficient mice had impaired CEnC wound healing. The WWTR1 transcript was highly expressed in healthy human CEnCs comparable to other genes implicated in FECD pathogenesis. Although WWTR1 mRNA expression was comparable between healthy and FECD-affected patients, WWTR1/TAZ protein concentrations were higher and localized to the nucleus surrounding guttae. No genetic associations were found in WWTR1 and FECD in a patient cohort compared to controls.
Conclusions: There are common phenotypic abnormalities seen between Wwtr1 deficient and FECD-affected patients, suggesting that Wwtr1 deficient mice could function as a murine model of late-onset FECD. Despite the lack of a genetic association between FECD and WWTR1, aberrant WWTR1/TAZ protein subcellular localization and degradation may play critical roles in the pathogenesis of FECD.
Conflict of interest statement
Disclosure:
Figures


References
-
- Dupont S, Morsut L, Aragona M, et al. .. Role of YAP/TAZ in mechanotransduction. Nature. 2011; 474: 179–183. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

