Pharmacokinetics of oral mannitol for bowel preparation for colonoscopy
- PMID: 37074807
- PMCID: PMC9579383
- DOI: 10.1111/cts.13373
Pharmacokinetics of oral mannitol for bowel preparation for colonoscopy
Abstract
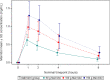
This study aimed to define the pharmacokinetics (PKs) of oral mannitol used as an osmotic laxative for bowel preparation for colonoscopy. The PKs of oral mannitol was evaluated in a substudy as part of a phase II dose-finding, international, multicenter, randomized, parallel-group, endoscopist-blinded study. Patients were randomly assigned to take 50, 100, or 150 g mannitol. Venous blood samples were drawn at baseline (T0), 1 h (T1), 2 h (T2), 4 h (T4), and 8 h (T8) after completion of mannitol self-administration. The mean mannitol plasma concentrations (mg/ml) were dose-dependent with a consistent difference among doses. The mean maximum concentration (Cmax) ± SD was 0.63 ± 0.15, 1.02 ± 0.28, and 1.36 ± 0.39 mg/ml, in the three dosage groups, respectively. The mean area under the curve from zero to infinity (AUC0-∞) was 2.667 ± 0.668, 4.992 ± 1.706, and 7.403 ± 3.472 mg/ml*h in the 50, 100, and 150 g mannitol dose groups, respectively. Bioavailability was similar in the three dose groups and was just over 20% (0.243 ± 0.073, 0.209 ± 0.081, and 0.228 ± 0.093 in the 50, 100, and 150 g mannitol dose groups, respectively). The present study showed that the bioavailability of oral mannitol is just over 20% and is similar for the three tested doses (50, 100, and 150 g). The linear increase in Cmax, AUC0-t8, and AUC0-∞ must be considered when choosing the oral mannitol dose for bowel preparation to avoid its systemic osmotic effects.
© 2022 NTC SRL and The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
A.O. is an employee of NTC. M.C. and B.M.C. are consultants with NTC. N.T.C. is developing a bowel cleansing preparation based on mannitol. All other authors declared no competing interests for this work.
Figures
References
-
- Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Gastroitest Endosc. 2015;81:31‐53. - PubMed
-
- Chapman W. The importance of adequate bowel cleansing for effective colonoscopy. Brit J Nursing. 2020;29:S3‐S8.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous