Metabolic switch in the aging astrocyte supported via integrative approach comprising network and transcriptome analyses
- PMID: 37074814
- PMCID: PMC10599759
- DOI: 10.18632/aging.204663
Metabolic switch in the aging astrocyte supported via integrative approach comprising network and transcriptome analyses
Abstract
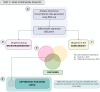
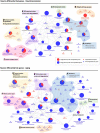
Dysregulated central-energy metabolism is a hallmark of brain aging. Supplying enough energy for neurotransmission relies on the neuron-astrocyte metabolic network. To identify genes contributing to age-associated brain functional decline, we formulated an approach to analyze the metabolic network by integrating flux, network structure and transcriptomic databases of neurotransmission and aging. Our findings support that during brain aging: (1) The astrocyte undergoes a metabolic switch from aerobic glycolysis to oxidative phosphorylation, decreasing lactate supply to the neuron, while the neuron suffers intrinsic energetic deficit by downregulation of Krebs cycle genes, including mdh1 and mdh2 (Malate-Aspartate Shuttle); (2) Branched-chain amino acid degradation genes were downregulated, identifying dld as a central regulator; (3) Ketone body synthesis increases in the neuron, while the astrocyte increases their utilization, in line with neuronal energy deficit in favor of astrocytes. We identified candidates for preclinical studies targeting energy metabolism to prevent age-associated cognitive decline.
Keywords: astrocyte; brain aging; flux balance analysis; network centrality; neuron.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

