Photothermal Perylene Bisimide Hydrogels
- PMID: 37074872
- PMCID: PMC10946608
- DOI: 10.1002/chem.202300663
Photothermal Perylene Bisimide Hydrogels
Abstract
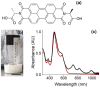
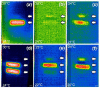
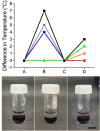
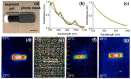
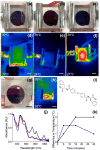
Gels formed using a perylene bisimide (PBI) as a low molecular weight gelator can show the photothermal effect. Formation of the PBI radical anion results in new absorption bands forming, meaning that subsequent irradiation with a wavelength of light overlapping with the new absorption band leads to heating of the gel. This approach can be used to heat the gel, as well as the surrounding milieu. We show how we can use electrochemical methods as well as multicomponent systems to form the radical anion without the need for UV light, and how we can use the photothermal effect to induce phase transitions in the solutions above the gels by exploiting photothermal behavior.
Keywords: PBI; gel; multicomponent; photothermal; radical anion.
© 2023 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Bian W., Wang Y., Pan Z., Chen N., Li X., Wong W.-L., Liu X., He Y., Zhang K., Lu Y.-J., ACS Appl. Nano Mater. 2021, 4, 11353–11385.
-
- Huang X., El-Sayed I. H., Qian W., El-Sayed M. A., J. Am. Chem. Soc. 2006, 128, 2115–2120. - PubMed
-
- Zhao L., Liu Y., Xing R., Yan X., Angew. Chem. Int. Ed. 2020, 59, 3793–3801; - PubMed
- Angew. Chem. 2020, 132, 3821–3829.

