BBB opening with focused ultrasound in nonhuman primates and Parkinson's disease patients: Targeted AAV vector delivery and PET imaging
- PMID: 37075119
- PMCID: PMC10115413
- DOI: 10.1126/sciadv.adf4888
BBB opening with focused ultrasound in nonhuman primates and Parkinson's disease patients: Targeted AAV vector delivery and PET imaging
Abstract
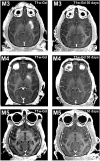
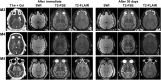
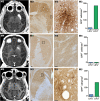
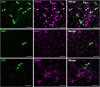
Intracerebral vector delivery in nonhuman primates has been a major challenge. We report successful blood-brain barrier opening and focal delivery of adeno-associated virus serotype 9 vectors into brain regions involved in Parkinson's disease using low-intensity focus ultrasound in adult macaque monkeys. Openings were well tolerated with generally no associated abnormal magnetic resonance imaging signals. Neuronal green fluorescent protein expression was observed specifically in regions with confirmed blood-brain barrier opening. Similar blood-brain barrier openings were safely demonstrated in three patients with Parkinson's disease. In these patients and in one monkey, blood-brain barrier opening was followed by 18F-Choline uptake in the putamen and midbrain regions based on positron emission tomography. This indicates focal and cellular binding of molecules that otherwise would not enter the brain parenchyma. The less-invasive nature of this methodology could facilitate focal viral vector delivery for gene therapy and might allow early and repeated interventions to treat neurodegenerative disorders.
Figures


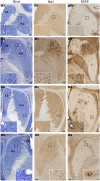
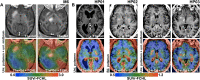
Comment in
-
Ultrasound lets gene therapy into the brain.Nat Rev Neurol. 2023 Jun;19(6):326. doi: 10.1038/s41582-023-00816-z. Nat Rev Neurol. 2023. PMID: 37138186 No abstract available.
References
-
- G. Deuschl, E. Beghi, F. Fazekas, T. Varga, K. A. Christoforidi, E. Sipido, C. L. Bassetti, T. Vos, V. L. Feigin, The burden of neurological diseases in Europe: An analysis for the Global Burden of Disease Study 2017. Lancet Public Health 5, e551–e567 (2020). - PubMed
-
- A. Akhtar, A. Andleeb, T. S. Waris, M. Bazzar, A.-R. Moradi, N. R. Awan, M. Yar, Neurodegenerative diseases and effective drug delivery: A review of challenges and novel therapeutics. J. Control. Release 330, 1152–1167 (2021). - PubMed
-
- C. N. Bedbrook, B. E. Deverman, V. Gradinaru, Viral strategies for targeting the central and peripheral nervous systems. Annu. Rev. Neurosci. 41, 323–348 (2018). - PubMed

