An influenza hemagglutinin stem nanoparticle vaccine induces cross-group 1 neutralizing antibodies in healthy adults
- PMID: 37075129
- PMCID: PMC10619166
- DOI: 10.1126/scitranslmed.ade4790
An influenza hemagglutinin stem nanoparticle vaccine induces cross-group 1 neutralizing antibodies in healthy adults
Abstract
Influenza vaccines could be improved by platforms inducing cross-reactive immunity. Immunodominance of the influenza hemagglutinin (HA) head in currently licensed vaccines impedes induction of cross-reactive neutralizing stem-directed antibodies. A vaccine without the variable HA head domain has the potential to focus the immune response on the conserved HA stem. This first-in-human dose-escalation open-label phase 1 clinical trial (NCT03814720) tested an HA stabilized stem ferritin nanoparticle vaccine (H1ssF) based on the H1 HA stem of A/New Caledonia/20/1999. Fifty-two healthy adults aged 18 to 70 years old enrolled to receive either 20 μg of H1ssF once (n = 5) or 60 μg of H1ssF twice (n = 47) with a prime-boost interval of 16 weeks. Thirty-five (74%) 60-μg dose participants received the boost, whereas 11 (23%) boost vaccinations were missed because of public health restrictions in the early stages of the COVID-19 pandemic. The primary objective of this trial was to evaluate the safety and tolerability of H1ssF, and the secondary objective was to evaluate antibody responses after vaccination. H1ssF was safe and well tolerated, with mild solicited local and systemic reactogenicity. The most common symptoms included pain or tenderness at the injection site (n = 10, 19%), headache (n = 10, 19%), and malaise (n = 6, 12%). We found that H1ssF elicited cross-reactive neutralizing antibodies against the conserved HA stem of group 1 influenza viruses, despite previous H1 subtype head-specific immunity. These responses were durable, with neutralizing antibodies observed more than 1 year after vaccination. Our results support this platform as a step forward in the development of a universal influenza vaccine.
Conflict of interest statement
Figures



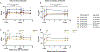
References
-
- Centers for Disease Control and Prevention, Disease Burden of Flu (Centers for Disease Control and Prevention, 2022); www.cdc.gov/flu/about/burden/index.html.
-
- Wiley DC, Skehel JJ, The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu. Rev. Biochem. 56, 365–394 (1987). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

