Leaflet by Leaflet Synergistic Effects of Antimicrobial Peptides on Bacterial and Mammalian Membrane Models
- PMID: 37075204
- PMCID: PMC10150393
- DOI: 10.1021/acs.jpclett.3c00119
Leaflet by Leaflet Synergistic Effects of Antimicrobial Peptides on Bacterial and Mammalian Membrane Models
Abstract
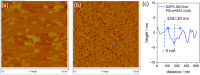
Antimicrobial peptides (AMPs) offer significant hope in the fight against antibiotic resistance. Operating via a mechanism different from that of antibiotics, they target the microbial membrane and ideally should damage it without impacting mammalian cells. Here, the interactions of two AMPs, magainin 2 and PGLa, and their synergistic effects on bacterial and mammalian membrane models were studied using electrochemical impedance spectroscopy, atomic force microscopy (AFM), and fluorescence correlation spectroscopy. Toroidal pore formation was observed by AFM when the two AMPs were combined, while individually AMP effects were confined to the exterior leaflet of the bacterial membrane analogue. Using microcavity-supported lipid bilayers, the diffusivity of each bilayer leaflet could be studied independently, and we observed that combined, the AMPs penetrate both leaflets of the bacterial model but individually each peptide had a limited impact on the proximal leaflet of the bacterial model. The impact of AMPs on a ternary, mammalian mimetic membrane was much weaker.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Heterodimer and pore formation of magainin 2 and PGLa: The anchoring and tilting of peptides in lipid bilayers.Biochim Biophys Acta Biomembr. 2020 Jul 1;1862(7):183305. doi: 10.1016/j.bbamem.2020.183305. Epub 2020 Apr 13. Biochim Biophys Acta Biomembr. 2020. PMID: 32298679
-
Magainin 2-PGLa Interactions in Membranes - Two Peptides that Exhibit Synergistic Enhancement of Antimicrobial Activity.Curr Top Med Chem. 2016;16(1):65-75. doi: 10.2174/1568026615666150703115701. Curr Top Med Chem. 2016. PMID: 26139118
-
Effects of the peptide Magainin H2 on Supported Lipid Bilayers studied by different biophysical techniques.Biochim Biophys Acta Biomembr. 2018 Dec;1860(12):2635-2643. doi: 10.1016/j.bbamem.2018.10.003. Epub 2018 Oct 4. Biochim Biophys Acta Biomembr. 2018. PMID: 30292399
-
The potential of AFM in studying the role of the nanoscale amphipathic nature of (lipo)-peptides interacting with lipid bilayers.Nanotechnology. 2022 Aug 1;33(43). doi: 10.1088/1361-6528/ac80c9. Nanotechnology. 2022. PMID: 35830770 Review.
-
SFG studies on interactions between antimicrobial peptides and supported lipid bilayers.Biochim Biophys Acta. 2006 Sep;1758(9):1257-73. doi: 10.1016/j.bbamem.2006.01.017. Epub 2006 Feb 17. Biochim Biophys Acta. 2006. PMID: 16524559 Review.
Cited by
-
Triplet-Triplet Annihilation Upconverting Liposomes: Mechanistic Insights into the Role of Membranes in Two-Dimensional TTA-UC.ACS Appl Mater Interfaces. 2024 Jun 5;16(22):29324-29337. doi: 10.1021/acsami.4c00990. Epub 2024 May 22. ACS Appl Mater Interfaces. 2024. PMID: 38776974 Free PMC article.
-
Hybrid bio-nanoporous peptide loaded-polymer platforms with anticancer and antibacterial activities.Nanoscale Adv. 2024 Feb 27;6(8):2038-2058. doi: 10.1039/d3na00947e. eCollection 2024 Apr 16. Nanoscale Adv. 2024. PMID: 38633049 Free PMC article.
-
Triplet-Triplet Annihilation Upconversion Is Impeded in Liposomes that Prevent Sensitizer and Annihilator Co-Confinement.J Phys Chem B. 2025 Jun 26;129(25):6220-6232. doi: 10.1021/acs.jpcb.5c01826. Epub 2025 Jun 12. J Phys Chem B. 2025. PMID: 40501149 Free PMC article.
-
The Evaluation of Teleost-Derived Antimicrobial Peptides Against Neisseria gonorrhoeae.Cureus. 2024 Mar 29;16(3):e57168. doi: 10.7759/cureus.57168. eCollection 2024 Mar. Cureus. 2024. PMID: 38681331 Free PMC article.
-
The Roadmap of Plant Antimicrobial Peptides Under Environmental Stress: From Farm to Bedside.Probiotics Antimicrob Proteins. 2024 Dec;16(6):2269-2304. doi: 10.1007/s12602-024-10354-9. Epub 2024 Sep 3. Probiotics Antimicrob Proteins. 2024. PMID: 39225894 Review.
References
-
- Cama J.; Leszczynski R.; Tang P. K.; Khalid A.; Lok V.; Dowson C. G.; Ebata A. To Push or to Pull? In a Post-COVID World, Supporting and Incentivizing Antimicrobial Drug Development Must Become a Governmental Priority. ACS Infect. Dis. 2021, 7, 2029–2042. 10.1021/acsinfecdis.0c00681. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

