Nutrient-sensing AgRP neurons relay control of liver autophagy during energy deprivation
- PMID: 37075752
- PMCID: PMC10173804
- DOI: 10.1016/j.cmet.2023.03.019
Nutrient-sensing AgRP neurons relay control of liver autophagy during energy deprivation
Abstract
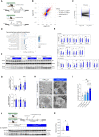
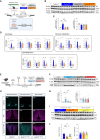
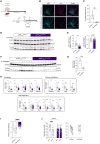
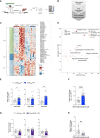
Autophagy represents a key regulator of aging and metabolism in sensing energy deprivation. We find that fasting in mice activates autophagy in the liver paralleled by activation of hypothalamic AgRP neurons. Optogenetic and chemogenetic activation of AgRP neurons induces autophagy, alters phosphorylation of autophagy regulators, and promotes ketogenesis. AgRP neuron-dependent induction of liver autophagy relies on NPY release in the paraventricular nucleus of the hypothalamus (PVH) via presynaptic inhibition of NPY1R-expressing neurons to activate PVHCRH neurons. Conversely, inhibiting AgRP neurons during energy deprivation abrogates induction of hepatic autophagy and rewiring of metabolism. AgRP neuron activation increases circulating corticosterone concentrations, and reduction of hepatic glucocorticoid receptor expression attenuates AgRP neuron-dependent activation of hepatic autophagy. Collectively, our study reveals a fundamental regulatory principle of liver autophagy in control of metabolic adaptation during nutrient deprivation.
Keywords: AgRP neurons; CRH neurons; HPA axis; NPY1R; autophagy; corticosterone; hypothalamus; liver metabolism; non-cell autonomous; short-term fasting.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




Comment in
-
Remote control of autophagy and metabolism in the liver.Cell Metab. 2023 May 2;35(5):725-727. doi: 10.1016/j.cmet.2023.04.009. Cell Metab. 2023. PMID: 37137284
References
-
- Perez-Riverol Y., Csordas A., Bai J., Bernal-Llinares M., Hewapathirana S., Kundu D.J., Inuganti A., Griss J., Mayer G., Eisenacher M., et al. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res. 2019;47:D442–D450. doi: 10.1093/nar/gky1106. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

