FFAR4 regulates cardiac oxylipin balance to promote inflammation resolution in HFpEF secondary to metabolic syndrome
- PMID: 37075982
- PMCID: PMC10209340
- DOI: 10.1016/j.jlr.2023.100374
FFAR4 regulates cardiac oxylipin balance to promote inflammation resolution in HFpEF secondary to metabolic syndrome
Abstract
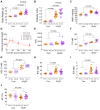
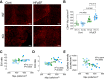
Heart failure with preserved ejection fraction (HFpEF) is a complex clinical syndrome, but a predominant subset of HFpEF patients has metabolic syndrome (MetS). Mechanistically, systemic, nonresolving inflammation associated with MetS might drive HFpEF remodeling. Free fatty acid receptor 4 (Ffar4) is a GPCR for long-chain fatty acids that attenuates metabolic dysfunction and resolves inflammation. Therefore, we hypothesized that Ffar4 would attenuate remodeling in HFpEF secondary to MetS (HFpEF-MetS). To test this hypothesis, mice with systemic deletion of Ffar4 (Ffar4KO) were fed a high-fat/high-sucrose diet with L-NAME in their water to induce HFpEF-MetS. In male Ffar4KO mice, this HFpEF-MetS diet induced similar metabolic deficits but worsened diastolic function and microvascular rarefaction relative to WT mice. Conversely, in female Ffar4KO mice, the diet produced greater obesity but no worsened ventricular remodeling relative to WT mice. In Ffar4KO males, MetS altered the balance of inflammatory oxylipins systemically in HDL and in the heart, decreasing the eicosapentaenoic acid-derived, proresolving oxylipin 18-hydroxyeicosapentaenoic acid (18-HEPE), while increasing the arachidonic acid-derived, proinflammatory oxylipin 12-hydroxyeicosatetraenoic acid (12-HETE). This increased 12-HETE/18-HEPE ratio reflected a more proinflammatory state both systemically and in the heart in male Ffar4KO mice and was associated with increased macrophage numbers in the heart, which in turn correlated with worsened ventricular remodeling. In summary, our data suggest that Ffar4 controls the proinflammatory/proresolving oxylipin balance systemically and in the heart to resolve inflammation and attenuate HFpEF remodeling.
Keywords: 18-hydroxyeicosapentaenoic acid; free fatty acid receptor 4 (Ffar4); heart failure preserved ejection fraction (HFpEF); inflammation; lipidomics; metabolic syndrome; obesity; omega-3 fatty acids; phospholipase a2.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures






References
-
- Dunlay S.M., Roger V.L., Redfield M.M. Epidemiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2017;14:591–602. - PubMed
-
- Steinberg B.A., Zhao X., Heidenreich P.A., Peterson E.D., Bhatt D.L., Cannon C.P., et al. Get With the Guidelines Scientific Advisory, and Investigators Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126:65–75. - PubMed
-
- Anker S.D., Butler J., Filippatos G., Ferreira J.P., Bocchi E., Bohm M., et al. Empagliflozin in heart failure with a preserved ejection fraction. N. Engl. J. Med. 2021;385:1451–1461. - PubMed
-
- Solomon S.D., McMurray J.J.V., Claggett B., de Boer R.A., DeMets D., Hernandez A.F., et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N. Engl. J. Med. 2022;387:1089–1098. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

