Assessing the impact of digital patient monitoring on health outcomes and healthcare resource usage in addition to the feasibility of its combination with at-home treatment, in participants receiving systemic anticancer treatment in clinical practice: protocol for an interventional, open-label, multicountry platform study (ORIGAMA)
- PMID: 37076159
- PMCID: PMC10124208
- DOI: 10.1136/bmjopen-2022-063242
Assessing the impact of digital patient monitoring on health outcomes and healthcare resource usage in addition to the feasibility of its combination with at-home treatment, in participants receiving systemic anticancer treatment in clinical practice: protocol for an interventional, open-label, multicountry platform study (ORIGAMA)
Abstract
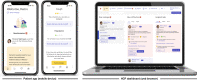
Introduction: Digital patient monitoring (DPM) tools can enable more effective clinical care and improved patient outcomes in cancer. However, their broad adoption requires ease of use and demonstration of real-world clinical utility/impact. ORIGAMA (MO42720) is an interventional, open-label, multicountry platform study investigating the clinical utility of DPM tools and specific treatments. ORIGAMA will begin with two cohorts that aim to assess the impact of the atezolizumab-specific Roche DPM Module (hosted on the Kaiku Health DPM platform (Helsinki, Finland)) on health outcomes and healthcare resource usage, and its feasibility to support at-home treatment administration, in participants receiving systemic anticancer treatment. Other digital health solutions may be added to future cohorts.
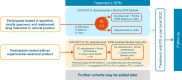
Methods and analysis: In Cohort A, participants with metastatic non-small cell lung cancer (NSCLC), extensive-stage SCLC or Child Pugh A unresectable hepatocellular carcinoma will be randomised to a locally approved anticancer regimen containing intravenous atezolizumab (TECENTRIQ, F. Hoffmann-La Roche Ltd/Genentech) and local standard-of-care support, with/without the Roche DPM Module. Cohort B will assess the feasibility of the Roche DPM Module in supporting administration of three cycles of subcutaneous atezolizumab (1875 mg; Day 1 of each 21-day cycle) in the hospital, followed by 13 cycles at home by a healthcare professional (ie, flexible care), in participants with programmed cell-death ligand 1-positive, early-stage NSCLC. The primary endpoints are the mean difference in change of the participant-reported Total Symptom Interference Score at Week 12 from baseline (Cohort A) and flexible care adoption rate at Cycle 6 (Cohort B).
Ethics and dissemination: This study will be conducted according to the Declaration of Helsinki, and/or the applicable laws and regulations of the country in which the research is conducted, whichever affords the greater protection to the individual. The study received its first Ethics Committee approval in Spain in October 2022. Participants will provide written informed consent in a face-to-face setting. The results of this study will be presented at national and/or international congresses and disseminated via publication in peer-reviewed journals.
Trial registration number: NCT05694013.
Keywords: Information management; ONCOLOGY; Telemedicine.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: SI has acted in a consultancy and advisory role for Bristol-Myers Squibb, Roche and Merck Sharp & Dohme; has participated in a speaker bureau or provided expert testimony for Boehringer Ingelheim; is an employee at an institution that has received a research grant or funding from Roche; and has received travel and accommodation expenses from Boehringer Ingelheim, Merck Sharp & Dohme, Roche, Novartis and Kaiku Health. A-MB: has received honoraria for participation in an advisory board for Roche (Ireland), has received institutional research funding to Lung Cancer Europe (LuCE) from Amgen, AstraZeneca, Bayer, Blueprint Medicines, BMS, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Merck, MSD, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda and Thermo Fisher, has received institutional honoraria to LuCE from Janssen, and has participated in various meetings, presentations and advisory boards, on behalf of LuCE. BB, AB, and AYCS are employees of and have stocks/other ownership in F. Hoffmann-La Roche Ltd. ME has acted in a consultancy and advisory role for Roche, is an employee at an institution that has received research grants from Novartis, Roche, BMS and Kaiku Health, and has received travel and accommodation expenses from Vifor. SG and MM-O are employees of and have stocks/other ownership in F. Hoffmann-La Roche Ltd. MR has received grants or contracts from Bayer and Ipsen, has received consulting fees from Bayer, BMS, Roche, Ipsen, AstraZeneca, Eli Lilly, BTG and Universal DX, and has received payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events, from Bayer, BMS, Gilead Sciences, Eli Lilly, Roche and Eisai. MW has received research funding from Roche for conduct of the study. JA is an employee of and has stocks/other ownership in F. Hoffmann-La Roche Ltd.
Figures

References
-
- World Health Organization (WHO), International Agency for Research on Cancer (IARC) . Global cancer observatory. 2022. Available: https://gco.iarc.fr/
-
- Allemani C, Matsuda T, Di Carlo V, et al. . Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023–75. 10.1016/S0140-6736(17)33326-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
