Lessons from a century of apical dominance research
- PMID: 37076257
- PMCID: PMC10400159
- DOI: 10.1093/jxb/erad137
Lessons from a century of apical dominance research
Abstract
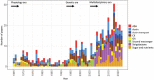
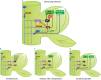
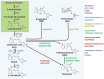
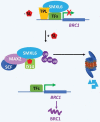
The process of apical dominance by which the apical bud/shoot tip of the plant inhibits the outgrowth of axillary buds located below has been studied for more than a century. Different approaches were used over time, with first the physiology era, the genetic era, and then the multidisciplinary era. During the physiology era, auxin was thought of as the master regulator of apical dominance acting indirectly to inhibit bud outgrowth via unknown secondary messenger(s). Potential candidates were cytokinin (CK) and abscisic acid (ABA). The genetic era with the screening of shoot branching mutants in different species revealed the existence of a novel carotenoid-derived branching inhibitor and led to the significant discovery of strigolactones (SLs) as a novel class of plant hormones. The re-discovery of the major role of sugars in apical dominance emerged from modern physiology experiments and involves ongoing work with genetic material affected in sugar signalling. As crops and natural selection rely on the emergent properties of networks such as this branching network, future work should explore the whole network, the details of which are critical but not individually sufficient to solve the 'wicked problems' of sustainable food supply and climate change.
Keywords: Apical dominance; auxin; axillary bud; cytokinins; genetics; physiology; shoot branching; strigolactones; sugars; tillering.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Conflict of interest statement
None declared
Figures
Similar articles
-
Auxin-independent effects of apical dominance induce changes in phytohormones correlated with bud outgrowth.Plant Physiol. 2023 May 31;192(2):1420-1434. doi: 10.1093/plphys/kiad034. Plant Physiol. 2023. PMID: 36690819 Free PMC article.
-
Change in Auxin and Cytokinin Levels Coincides with Altered Expression of Branching Genes during Axillary Bud Outgrowth in Chrysanthemum.PLoS One. 2016 Aug 24;11(8):e0161732. doi: 10.1371/journal.pone.0161732. eCollection 2016. PLoS One. 2016. PMID: 27557329 Free PMC article.
-
Roles of auxin in the inhibition of shoot branching in 'Dugan' fir.Tree Physiol. 2022 Jul 5;42(7):1411-1431. doi: 10.1093/treephys/tpac008. Tree Physiol. 2022. PMID: 35088089
-
Strigolactones, a novel class of plant hormone controlling shoot branching.C R Biol. 2010 Apr;333(4):344-9. doi: 10.1016/j.crvi.2010.01.012. Epub 2010 Mar 16. C R Biol. 2010. PMID: 20371109 Review.
-
Light Regulation of Axillary Bud Outgrowth Along Plant Axes: An Overview of the Roles of Sugars and Hormones.Front Plant Sci. 2019 Oct 18;10:1296. doi: 10.3389/fpls.2019.01296. eCollection 2019. Front Plant Sci. 2019. PMID: 31681386 Free PMC article. Review.
Cited by
-
Appropriate mowing can promote the growth of Anabasis aphylla through the auxin metabolism pathway.BMC Plant Biol. 2024 May 31;24(1):482. doi: 10.1186/s12870-024-05204-3. BMC Plant Biol. 2024. PMID: 38822275 Free PMC article.
-
Seven plant capacities to adapt to abiotic stress.J Exp Bot. 2023 Aug 17;74(15):4308-4323. doi: 10.1093/jxb/erad179. J Exp Bot. 2023. PMID: 37220077 Free PMC article. Review.
-
Strigolactone and karrikin receptors regulate phytohormone biosynthetic and catabolic processes.Plant Cell Rep. 2025 Feb 21;44(3):60. doi: 10.1007/s00299-025-03456-3. Plant Cell Rep. 2025. PMID: 39982558
-
Physiological and molecular mechanisms associated with potato tuber dormancy.J Exp Bot. 2024 Oct 16;75(19):6093-6109. doi: 10.1093/jxb/erae182. J Exp Bot. 2024. PMID: 38650389 Free PMC article. Review.
-
How do brassinosteroids fit in bud outgrowth models?J Exp Bot. 2024 Jan 1;75(1):13-16. doi: 10.1093/jxb/erad394. J Exp Bot. 2024. PMID: 37846132 Free PMC article.
References
-
- Abuauf H, Haider I, Jia K-P, Ablazov A, Mi J, Blilou I, Al-Babili S.. 2018. The Arabidopsis DWARF27 gene encodes an all-trans-/9-cis-β-carotene isomerase and is induced by auxin, abscisic acid and phosphate deficiency. Plant Science 277, 33–42. - PubMed
-
- Akiyama K, Matsuzaki K, Hayashi H.. 2005. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435, 824–827. - PubMed
-
- Alder A, Jamil M, Marzorati M, Bruno M, Vermathen M, Bigler P, Ghisla S, Bouwmeester H, Beyer P, Al-Babili S.. 2012. The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science 335, 1348–1351. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

