Endosidin 5 disruption of the Golgi apparatus and extracellular matrix secretion in the unicellular charophyte Penium margaritaceum
- PMID: 37076269
- PMCID: PMC10332397
- DOI: 10.1093/aob/mcad054
Endosidin 5 disruption of the Golgi apparatus and extracellular matrix secretion in the unicellular charophyte Penium margaritaceum
Abstract
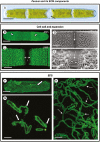
Background and aims: Endosidins are a group of low-molecular-weight compounds, first identified by 'chemical biology' screening assays, that have been used to target specific components of the endomembrane system. In this study, we employed multiple microscopy-based screening techniques to elucidate the effects of endosidin 5 (ES5) on the Golgi apparatus and the secretion of extracellular matrix (ECM) components in Penium margaritaceum. These effects were compared with those caused by treatments with brefeldin A and concanamycin A. Penium margaritaceum's extensive Golgi apparatus and endomembrane system make it an outstanding model organism for screening changes to the endomembrane system. Here we detail changes to the Golgi apparatus and secretion of ECM material caused by ES5.
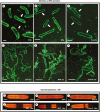
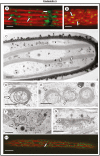
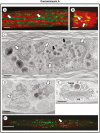
Methods: Changes to extracellular polymeric substance (EPS) secretion and cell wall expansion were screened using fluorescence microscopy. Confocal laser scanning microscopy and transmission electron microscopy were used to assess changes to the Golgi apparatus, the cell wall and the vesicular network. Electron tomography was also performed to detail the changes to the Golgi apparatus.
Key results: While other endosidins were able to impact EPS secretion and cell wall expansion, only ES5 completely inhibited EPS secretion and cell wall expansion over 24 h. Short treatments of ES5 resulted in displacement of the Golgi bodies from their typical linear alignment. The number of cisternae decreased per Golgi stack and trans face cisternae in-curled to form distinct elongate circular profiles. Longer treatment resulted in a transformation of the Golgi body to an irregular aggregate of cisternae. These alterations could be reversed by removal of ES5 and returning cells to culture.
Conclusions: ES5 alters secretion of ECM material in Penium by affecting the Golgi apparatus and does so in a markedly different way from other endomembrane inhibitors such as brefeldin A and concanamycin A.
Keywords: Charophytes; Golgi apparatus; brefeldin A; concanamycin A; endomembrane system; endosidin; secretion; tomography.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Annals of Botany Company.
Figures



Similar articles
-
Endomembrane architecture and dynamics during secretion of the extracellular matrix of the unicellular charophyte, Penium margaritaceum.J Exp Bot. 2020 Jun 11;71(11):3323-3339. doi: 10.1093/jxb/eraa039. J Exp Bot. 2020. PMID: 31974570 Free PMC article.
-
Callose deposition is essential for the completion of cytokinesis in the unicellular alga Penium margaritaceum.J Cell Sci. 2020 Oct 12;133(19):jcs249599. doi: 10.1242/jcs.249599. J Cell Sci. 2020. PMID: 32895244
-
The cortical cytoskeletal network and cell-wall dynamics in the unicellular charophycean green alga Penium margaritaceum.Ann Bot. 2014 Oct;114(6):1237-49. doi: 10.1093/aob/mcu013. Epub 2014 Mar 5. Ann Bot. 2014. PMID: 24603606 Free PMC article.
-
Penium margaritaceum: A Unicellular Model Organism for Studying Plant Cell Wall Architecture and Dynamics.Plants (Basel). 2014 Nov 18;3(4):543-58. doi: 10.3390/plants3040543. Plants (Basel). 2014. PMID: 27135519 Free PMC article. Review.
-
Perspectives on Golgi apparatus form and function.J Electron Microsc Tech. 1991 Jan;17(1):2-14. doi: 10.1002/jemt.1060170103. J Electron Microsc Tech. 1991. PMID: 1993935 Review.
Cited by
-
More than a passive barrier: algal cell walls play an active role in determining cell shape, cell size, and organelle morphology.J Exp Bot. 2025 Feb 25;76(4):899-903. doi: 10.1093/jxb/erae411. J Exp Bot. 2025. PMID: 39996292 Free PMC article.
-
Synergism of vesicle trafficking and cytoskeleton during regulation of plant growth and development: A mechanistic outlook.Heliyon. 2023 Nov 8;9(11):e21976. doi: 10.1016/j.heliyon.2023.e21976. eCollection 2023 Nov. Heliyon. 2023. Retraction in: Heliyon. 2025 Mar 18;11(8):e43207. doi: 10.1016/j.heliyon.2025.e43207. PMID: 38034654 Free PMC article. Retracted. Review.
-
Chemically induced phenotype plasticity in the unicellular zygnematophyte, Penium margaritaceum.Protoplasma. 2024 Nov;261(6):1233-1249. doi: 10.1007/s00709-024-01962-x. Epub 2024 Jul 5. Protoplasma. 2024. PMID: 38967680 Free PMC article.
References
-
- Boney AD. 1981. Mucilage: A ubiquitous algal attribute. British Phycological Journal 16: 115–132. doi:10.1080/00071618100650101. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

