Granulocyte activation markers in cerebrospinal fluid differentiate acute neuromyelitis spectrum disorder from multiple sclerosis
- PMID: 37076291
- PMCID: PMC10447383
- DOI: 10.1136/jnnp-2022-330796
Granulocyte activation markers in cerebrospinal fluid differentiate acute neuromyelitis spectrum disorder from multiple sclerosis
Abstract
Background: Granulocyte invasion into the brain is a pathoanatomical feature differentiating neuromyelitis optica spectrum disorder (NMOSD) from multiple sclerosis (MS). We aimed to determine whether granulocyte activation markers (GAM) in cerebrospinal fluid (CSF) can be used as a biomarker to distinguish NMOSD from MS, and whether levels associate with neurological impairment.
Methods: We quantified CSF levels of five GAM (neutrophil elastase, myeloperoxidase, neutrophil gelatinase-associated lipocalin, matrixmetalloproteinase-8, tissue inhibitor of metalloproteinase-1), as well as a set of inflammatory and tissue-destruction markers, known to be upregulated in NMOSD and MS (neurofilament light chain, glial fibrillary acidic protein, S100B, matrix metalloproteinase-9, intercellular adhesion molecule-1, vascular cellular adhesion molecule-1), in two cohorts of patients with mixed NMOSD and relapsing-remitting multiple sclerosis (RRMS).
Results: In acute NMOSD, GAM and adhesion molecules, but not the other markers, were higher than in RRMS and correlated with actual clinical disability scores. Peak GAM levels occurred at the onset of NMOSD attacks, while they were stably low in MS, allowing to differentiate the two diseases for ≤21 days from onset of clinical exacerbation. Composites of GAM provided area under the curve values of 0.90-0.98 (specificity of 0.76-1.0, sensitivity of 0.87-1.0) to differentiate NMOSD from MS, including all anti-aquaporin-4 protein (aAQP4)-antibody-negative patients who were untreated.
Conclusions: GAM composites represent a novel biomarker to reliably differentiate NMOSD from MS, including in aAQP4- NMOSD. The association of GAM with the degree of concurrent neurological impairment provides evidence for their pathogenic role, in turn suggesting them as potential drug targets in acute NMOSD.
Keywords: CSF; clinical neurology; molecular biology; multiple sclerosis; neuroimmunology.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: DL is Chief Medical Officer of GeNeuro. MW received speaker honoraria from Novartis Pharma, Chugai Pharmaceutical, Biogen Japan and Alexion. FP has received research grants from Janssen, Merck KGaA and UCB, and fees for serving on DMC in clinical trials with Chugai, Lundbeck and Roche, and preparation of witness report for Novartis. RF has received speaker fees for teaching and workshops from Biogen, Merck, Novartis, Roche, Teva and Alexion. For educational activities, courses or research, he has received unrestricted grants from Biogen, EMD Serono. JL is an employee of Quanterix. BE has received travel grants for ECTRIMS 2018 from Roche. KF has served on advisory boards and received speaker honoraria from Biogen, Roche and Merck and received research funds from Amicus. TM received speaker honoraria from Biogen Japan, Chugai Pharmaceutical, Alexion Pharmaceuticals, Novartis Pharma and Takeda Pharmaceutical. KM received speaker honoraria from Novartis Pharma, Chugai Pharmaceutical and Nihon Pharmaceutical. NI received grant support from Mitsubishi Tanabe Pharma, Osoegawa Neurology Clinic, Bayer Yakuhin and Japan Blood Products Organization and speaker honoraria from Novartis Pharma, Biogen Japan, Alexion, Mitsubishi Tanabe Pharma, Chugai Pharmaceutical, Teijin Pharma and Eisai. J-IK received research funds from Dainippon Sumitomo Pharma, Daiichi Sankyo, Mitsubishi Tanabe Pharma and Kyowa Kensetsukougyo, and consultancy fees, speaking fees and/or honoraria from Novartis Pharma, Mitsubishi Tanabe Pharma, CSL Behring, Biogen Japan, Teijin Health Care, the Takeda Pharmaceutical, Kyowa Kirin, Ono Pharmaceutical, Alexion Pharmaceuticals, Tsumura, Ricoh, EMC and Eisai. JO served on advisory boards for Roche and Merck. JK received speaker fees, research support, travel support and/or served on advisory boards by the Progressive MS Alliance, Swiss MS Society, Swiss National Research Foundation (320030_189140/1), University of Basel, Biogen, Celgene, Merck, Novartis, Octave Bioscience, Roche, Sanofi. No other disclosures were reported.
Figures
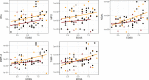
 ; s/c:
; s/c: ) corticosteroid pre-treatment; patients with (acute:
) corticosteroid pre-treatment; patients with (acute:  ; s/c:
; s/c: ) corticosteroid pre-treatment. Open symbols designate aAQP4− patients. The Spearman’s correlation analysis of all, acute and s/c cohorts of patients showed significant correlations with the EDSS score; note that intercellular adhesion molecule-1 and vascular cellular adhesion molecule-1 levels correlated as well with EDSS scores (see online supplemental table 3). EDSS, Expanded Disability Status Scale; MMP-8, matrix metalloproteinase 8; MPO, myeloperoxidase; nEla, neutrophil elastase; NGAL, neutrophil gelatinase-associated lipocalin; NMOSD, neuromyelitis optica spectrum disorder; TIMP-1, tissue inhibitor of metalloproteinase-1.
) corticosteroid pre-treatment. Open symbols designate aAQP4− patients. The Spearman’s correlation analysis of all, acute and s/c cohorts of patients showed significant correlations with the EDSS score; note that intercellular adhesion molecule-1 and vascular cellular adhesion molecule-1 levels correlated as well with EDSS scores (see online supplemental table 3). EDSS, Expanded Disability Status Scale; MMP-8, matrix metalloproteinase 8; MPO, myeloperoxidase; nEla, neutrophil elastase; NGAL, neutrophil gelatinase-associated lipocalin; NMOSD, neuromyelitis optica spectrum disorder; TIMP-1, tissue inhibitor of metalloproteinase-1.

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
