Association of Stages of Objective Memory Impairment With Incident Symptomatic Cognitive Impairment in Cognitively Normal Individuals
- PMID: 37076305
- PMCID: PMC10259282
- DOI: 10.1212/WNL.0000000000207276
Association of Stages of Objective Memory Impairment With Incident Symptomatic Cognitive Impairment in Cognitively Normal Individuals
Abstract
Background and objectives: Increasing evidence indicates that a subset of cognitively normal individuals has subtle cognitive impairment at baseline. We sought to identify them using the Stages of Objective Memory Impairment (SOMI) system. Symptomatic cognitive impairment was operationalized by a Clinical Dementia Rating (CDR) ≥0.5. We hypothesized that incident impairment would be higher for participants with subtle retrieval impairment (SOMI-1), higher still for those with moderate retrieval impairment (SOMI-2), and highest for those with storage impairment (SOMI-3/4) after adjusting for demographics and APOE ε4 status. A secondary objective was to determine whether including biomarkers of β-amyloid, tau pathology, and neurodegeneration in the models affect prediction. We hypothesized that even after adjusting for in vivo biomarkers, SOMI would remain a significant predictor of time to incident symptomatic cognitive impairment.
Methods: Among 969 cognitively normal participants, defined by a CDR = 0, from the Knight Alzheimer Disease Research Center, SOMI stage was determined from their baseline Free and Cued Selective Reminding Test scores, 555 had CSF and structural MRI measures and comprised the biomarker subgroup, and 144 of them were amyloid positive. Cox proportional hazards models tested associations of SOMI stages at baseline and biomarkers with time to incident cognitive impairment defined as the transition to CDR ≥0.5.
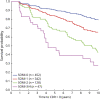
Results: Among all participants, the mean age was 69.35 years, 59.6% were female, and mean follow-up was 6.36 years. Participants in SOMI-1-4 had elevated hazard ratios for the transition from normal to impaired cognition in comparison with those who were SOMI-0 (no memory impairment). Individuals in SOMI-1 (mildly impaired retrieval) and SOMI-2 (moderately impaired retrieval) were at nearly double the risk of clinical progression compared with persons with no memory problems. When memory storage impairment emerges (SOMI-3/4), the hazard ratio for clinical progression increased approximately 3 times. SOMI stage remained an independent predictor of incident cognitive impairment after adjusting for all biomarkers.
Discussion: SOMI predicts the transition from normal cognition to incident symptomatic cognitive impairment (CDR ≥0.5). The results support the use of SOMI to identify those cognitively normal participants most likely to develop incident cognitive impairment who can then be referred for biomarker screening.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Conflict of interest statement
E. Grober receives a small royalty for commercial use of the Free and Cued Selective Reminding Test with Immediate Recall (FCSRT + IR). The test is available at no cost to researchers and clinicians. The Albert Einstein College of Medicines holds the copyright for the test. Inquiries should be addressed to Dr. Nilam Sinha in the Office of Biotechnology and Business Development (
Figures
Similar articles
-
Assessing Stages of Objective Memory Impairment and neuroimaging as risk factors of incident cognitive impairment.J Int Neuropsychol Soc. 2025 Aug 22:1-9. doi: 10.1017/S1355617725101240. Online ahead of print. J Int Neuropsychol Soc. 2025. PMID: 40842062
-
Associations of Stages of Objective Memory Impairment with Cerebrospinal Fluid and Neuroimaging Biomarkers of Alzheimer's Disease.J Prev Alzheimers Dis. 2023;10(1):112-119. doi: 10.14283/jpad.2022.98. J Prev Alzheimers Dis. 2023. PMID: 36641615 Free PMC article.
-
Stages of Objective Memory Impairment Predict Alzheimer's Disease Neuropathology: Comparison with the Clinical Dementia Rating Scale-Sum of Boxes.J Alzheimers Dis. 2021;80(1):185-195. doi: 10.3233/JAD-200946. J Alzheimers Dis. 2021. PMID: 33492286 Free PMC article.
-
Incident cognitive impairment: longitudinal changes in molecular, structural and cognitive biomarkers.Brain. 2018 Nov 1;141(11):3233-3248. doi: 10.1093/brain/awy244. Brain. 2018. PMID: 30304397 Free PMC article.
-
Amyloid and Tau Prediction of Cognitive and Functional Decline in Unimpaired Older Individuals: Longitudinal Data from the A4 and LEARN Studies.J Prev Alzheimers Dis. 2024;11(4):802-813. doi: 10.14283/jpad.2024.122. J Prev Alzheimers Dis. 2024. PMID: 39044488 Free PMC article. Clinical Trial.
Cited by
-
Cerebrospinal fluid proteomic profiling of cognitively unimpaired individuals with suspected non-Alzheimer's disease pathophysiology.Brain Commun. 2025 Jun 20;7(4):fcaf253. doi: 10.1093/braincomms/fcaf253. eCollection 2025. Brain Commun. 2025. PMID: 40641595 Free PMC article.
-
CSF proteomic profiles of neurodegeneration biomarkers in Alzheimer's disease.Alzheimers Dement. 2024 Sep;20(9):6205-6220. doi: 10.1002/alz.14103. Epub 2024 Jul 6. Alzheimers Dement. 2024. PMID: 38970402 Free PMC article.
-
Influence of daily life and health profile in subtle cognitive decline of women residing in Spanish religious communities: DeCo religious orders study.Front Public Health. 2024 Jul 17;12:1395877. doi: 10.3389/fpubh.2024.1395877. eCollection 2024. Front Public Health. 2024. PMID: 39086806 Free PMC article.
-
The effects of musicality on brain network topology in the context of Alzheimer's disease and memory decline.Imaging Neurosci (Camb). 2024 Aug 5;2:imag-2-00248. doi: 10.1162/imag_a_00248. eCollection 2024. Imaging Neurosci (Camb). 2024. PMID: 40800535 Free PMC article.
-
Free Recall Outperforms Story Recall in Associations with Plasma Biomarkers in Preclinical Alzheimer Disease.J Prev Alzheimers Dis. 2024;11(6):1696-1702. doi: 10.14283/jpad.2024.130. J Prev Alzheimers Dis. 2024. PMID: 39559880 Free PMC article.
References
-
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412-2414. - PubMed
-
- Aisen PS, Zhou J, Irizarry MC, et al. . AHEAD 3‐45 study design: a global study to evaluate the efficacy and safety of treatment with BAN2401 for 216 weeks in preclinical Alzheimer's disease with intermediate amyloid (A3 trial) and elevated amyloid (A45 trial) Clinical trial design and implementation. Alzheimers Dement. 2020;16(S9):e044511.
Publication types
MeSH terms
Substances
Grants and funding
- P30 AG066444/AG/NIA NIH HHS/United States
- R01 AG048642/AG/NIA NIH HHS/United States
- U24 NS113847/NS/NINDS NIH HHS/United States
- R01 AG062622/AG/NIA NIH HHS/United States
- UG3 FD006795/FD/FDA HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- P01 AG026276/AG/NIA NIH HHS/United States
- R56 AG057548/AG/NIA NIH HHS/United States
- U19 AG024904/AG/NIA NIH HHS/United States
- R01 AG060933/AG/NIA NIH HHS/United States
- RF1 AG054548/AG/NIA NIH HHS/United States
- K23 AG063993/AG/NIA NIH HHS/United States
- R21 AG056920/AG/NIA NIH HHS/United States
- U10 NS077308/NS/NINDS NIH HHS/United States
- P01 AG003949/AG/NIA NIH HHS/United States
- RF1 AG057531/AG/NIA NIH HHS/United States
