Prospective Multicenter Validation of a Simple Blood Test for the Diagnosis of Glut1 Deficiency Syndrome
- PMID: 37076312
- PMCID: PMC10256121
- DOI: 10.1212/WNL.0000000000207296
Prospective Multicenter Validation of a Simple Blood Test for the Diagnosis of Glut1 Deficiency Syndrome
Abstract
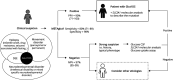
Background and objective: GLUT1 deficiency syndrome (Glut1DS) is a treatable neurometabolic disease that causes a wide range of neurologic symptoms in children and adults. However, its diagnosis relies on an invasive test, that is, a lumbar puncture (LP) to measure glycorrhachia, and sometimes complex molecular analyses of the SLC2A1 gene. This procedure limits the number of patients able to receive the standard of care. We wished to validate the diagnostic performance of METAglut1, a simple blood test that quantifies GLUT1 on the erythrocyte surface.
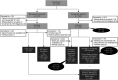
Methods: We performed a multicenter validation study in France, involving 33 centers. We studied 2 patient cohorts: a prospective cohort consisting of patients with a clinical suspicion of Glut1DS explored through the reference strategy, that is, LP and analyses of the SLC2A1 gene, and a retrospective cohort that included patients previously diagnosed with Glut1DS. All patients were blind-tested with METAglut1.
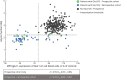
Results: We analyzed 428 patients in the prospective cohort, including 15 patients newly diagnosed with Glut1DS, and 67 patients in the retrospective cohort. METAglut1 was 80% sensitive and >99% specific for the diagnosis of Glut1DS. Concordance analyses showed a substantial agreement between METAglut1 and glycorrhachia. In the prospective cohort, the positive predictive value of METAglut1 was slightly higher than that of glycorrhachia. METAglut1 succeeded to identify patients with Glut1DS with SCL2A1 mosaicism and variants of unknown significance.
Discussion: METAglut1 is an easily performed, robust, and noninvasive diagnostic test for the diagnosis of Glut1DS, which allows wide screening of children and adults, including those with atypical forms of this treatable condition.
Classification of evidence: This study provides Class I evidence that a positive METAglut1 test accurately distinguishes patients with suspected GLUT1 deficiency syndrome from other neurologic syndromes as compared with invasive and genetic testing.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Conflict of interest statement
F. Mochel serves on the scientific advisory board of Metafora biosystems; METAFORA biosystems is a startup that developed METAglut1; D. Gras reports no disclosures relevant to the manuscript; MP. Luton reports no disclosures relevant to the manuscript; M. Nizou is an employee at Metafora biosystems; D. Giovannini reports no disclosures relevant to the manuscript; C. Delattre reports no disclosures relevant to the manuscript; M. Aubart reports no disclosures relevant to the manuscript; M. Barth reports no disclosures relevant to the manuscript; A. De Saint-Martin reports no disclosures relevant to the manuscript; D. Doummar reports no disclosures relevant to the manuscript; N. Essid reports no disclosures relevant to the manuscript; A. Garros reports no disclosures relevant to the manuscript; C. Hachon-Le Camus reports no disclosures relevant to the manuscript; C. Hoebeke reports no disclosures relevant to the manuscript; S. Nguyen The Tich reports no disclosures relevant to the manuscript; M. Périvier reports no disclosures relevant to the manuscript; S. Rivera reports no disclosures relevant to the manuscript; A. Rolland reports no disclosures relevant to the manuscript; A. Roubertie reports no disclosures relevant to the manuscript; C. Sarret reports no disclosures relevant to the manuscript; C. Sevin reports no disclosures relevant to the manuscript; D. Ville reports no disclosures relevant to the manuscript; M. Sitbon is the inventor of a patent describing the use of the H2RBD ligand for the evaluation of GLUT1 expression; he is the cofounder of Metafora biosystems and head of the scientific advisory board; JM. Costa is biologist at Cerba Healthcare, a testing laboratory implementing the Metaglut1 assay for the routine; R. Pons reports no disclosures relevant to the manuscript; A. Garcia-Cazorla reports no disclosures relevant to the manuscript; S. Vuillaumier-Barrot reports no disclosures relevant to the manuscript; V. Petit is a cofounder and CEO of Metafora biosystems; O. Boespflug-Tanguy reports no disclosures relevant to the manuscript; and D. C. De Vivo serves on the scientific advisory board of Metafora biosystems. Go to
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
