In vitro PCR verification that lysozyme inhibits nucleic acid replication and transcription
- PMID: 37076576
- PMCID: PMC10115842
- DOI: 10.1038/s41598-023-33228-6
In vitro PCR verification that lysozyme inhibits nucleic acid replication and transcription
Abstract
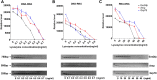
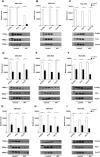
Lysozyme can kill bacteria by its enzymatic activity or through a mechanism involving its cationic nature, which can facilitate electrostatic interactions with the viral capsid, the negatively charged parts of nucleic acids, and polymerase, so binding to nucleic acids may be another biological function of lysozyme. Here, PCR was used as a research tool to detect the effects of lysozyme on the replication and transcription of nucleic acids after treatment in different ways. We found that lysozyme and its hydrolysate can enter cells and inhibit PCR to varying degrees in vitro, and degraded lysozyme inhibited nucleic acid replication more effectively than intact lysozyme. The inhibition of lysozyme may be related to polymerase binding, and the sensitivity of different polymerases to lysozyme is inconsistent. Our findings provide a theoretical basis for further explaining the pharmacological effects of lysozyme, such as antibacterial, antiviral, anticancer, and immune regulatory activities, and directions for the development of new pharmacological effects of lysozyme and its metabolites.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

