The Smc5/6 complex is a DNA loop-extruding motor
- PMID: 37076626
- PMCID: PMC10132971
- DOI: 10.1038/s41586-023-05963-3
The Smc5/6 complex is a DNA loop-extruding motor
Abstract
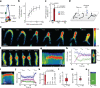
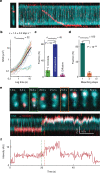
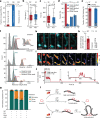
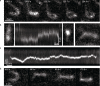
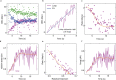
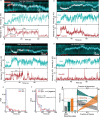
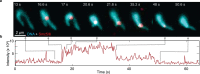
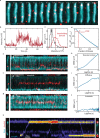
Structural maintenance of chromosomes (SMC) protein complexes are essential for the spatial organization of chromosomes1. Whereas cohesin and condensin organize chromosomes by extrusion of DNA loops, the molecular functions of the third eukaryotic SMC complex, Smc5/6, remain largely unknown2. Using single-molecule imaging, we show that Smc5/6 forms DNA loops by extrusion. Upon ATP hydrolysis, Smc5/6 reels DNA symmetrically into loops at a force-dependent rate of one kilobase pair per second. Smc5/6 extrudes loops in the form of dimers, whereas monomeric Smc5/6 unidirectionally translocates along DNA. We also find that the subunits Nse5 and Nse6 (Nse5/6) act as negative regulators of loop extrusion. Nse5/6 inhibits loop-extrusion initiation by hindering Smc5/6 dimerization but has no influence on ongoing loop extrusion. Our findings reveal functions of Smc5/6 at the molecular level and establish DNA loop extrusion as a conserved mechanism among eukaryotic SMC complexes.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Comment in
-
Smc5/6 caught in the act of loop extrusion.Nat Struct Mol Biol. 2023 May;30(5):567. doi: 10.1038/s41594-023-01007-6. Nat Struct Mol Biol. 2023. PMID: 37198271 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

