A somato-cognitive action network alternates with effector regions in motor cortex
- PMID: 37076628
- PMCID: PMC10172144
- DOI: 10.1038/s41586-023-05964-2
A somato-cognitive action network alternates with effector regions in motor cortex
Abstract
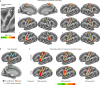
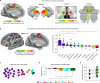
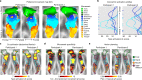
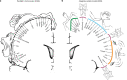
Motor cortex (M1) has been thought to form a continuous somatotopic homunculus extending down the precentral gyrus from foot to face representations1,2, despite evidence for concentric functional zones3 and maps of complex actions4. Here, using precision functional magnetic resonance imaging (fMRI) methods, we find that the classic homunculus is interrupted by regions with distinct connectivity, structure and function, alternating with effector-specific (foot, hand and mouth) areas. These inter-effector regions exhibit decreased cortical thickness and strong functional connectivity to each other, as well as to the cingulo-opercular network (CON), critical for action5 and physiological control6, arousal7, errors8 and pain9. This interdigitation of action control-linked and motor effector regions was verified in the three largest fMRI datasets. Macaque and pediatric (newborn, infant and child) precision fMRI suggested cross-species homologues and developmental precursors of the inter-effector system. A battery of motor and action fMRI tasks documented concentric effector somatotopies, separated by the CON-linked inter-effector regions. The inter-effectors lacked movement specificity and co-activated during action planning (coordination of hands and feet) and axial body movement (such as of the abdomen or eyebrows). These results, together with previous studies demonstrating stimulation-evoked complex actions4 and connectivity to internal organs10 such as the adrenal medulla, suggest that M1 is punctuated by a system for whole-body action planning, the somato-cognitive action network (SCAN). In M1, two parallel systems intertwine, forming an integrate-isolate pattern: effector-specific regions (foot, hand and mouth) for isolating fine motor control and the SCAN for integrating goals, physiology and body movement.
© 2023. The Author(s).
Conflict of interest statement
D.A.F., N.U.F.D. and N.A.S. have a financial interest in Turing Medical Inc. and may benefit financially if the company is successful in marketing FIRMM motion monitoring software products. A.N.V., D.A.F. and N.U.F.D. may receive royalty income based on FIRMM technology developed at Washington University School of Medicine and Oregon Health and Sciences University and licensed to Turing Medical Inc. D.A.F. and N.U.F.D. are co-founders of Turing Medical Inc. These potential conflicts of interest have been reviewed and are managed by Washington University School of Medicine, Oregon Health and Sciences University and the University of Minnesota. N.A.S. is now an employee of Turing Medical Inc. C.M.S receives research support from Sage Therapeutics. C.L. is listed as an inventor for Cornell University patent applications on neuroimaging biomarkers for depression that are pending or in preparation. C.L. has served as a scientific advisor or consultant to Compass Pathways PLC, Delix Therapeutics, Magnus Medical and Brainify.AI. The other authors declare no competing interests.
Figures
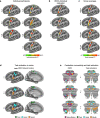
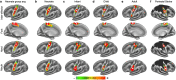
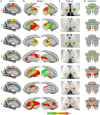

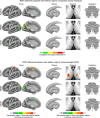
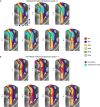
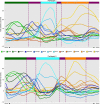
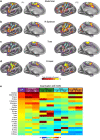
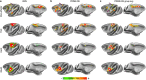
Comment in
-
A redrawn map for the human motor cortex.Nature. 2023 May;617(7960):253-254. doi: 10.1038/d41586-023-01350-0. Nature. 2023. PMID: 37076716 Free PMC article.
-
Fundamental principles of cortical organization reflected in a new study.Neuron. 2023 May 17;111(10):1524-1525. doi: 10.1016/j.neuron.2023.04.024. Epub 2023 May 3. Neuron. 2023. PMID: 37141890
References
-
- Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. doi: 10.1093/brain/60.4.389. - DOI
-
- Penfield, W. & Rasmussen, T. The Cerebral Cortex of Man; A Clinical Study of Localization of Function. xv, 248 (Macmillan, 1950).
Publication types
MeSH terms
Grants and funding
- K23 NS088590/NS/NINDS NIH HHS/United States
- R01 MH122389/MH/NIMH NIH HHS/United States
- T32 MH100019/MH/NIMH NIH HHS/United States
- R01 MH096773/MH/NIMH NIH HHS/United States
- R01 DA049547/DA/NIDA NIH HHS/United States
- R01 MH118362/MH/NIMH NIH HHS/United States
- R01 MH118388/MH/NIMH NIH HHS/United States
- R01 MH128177/MH/NIMH NIH HHS/United States
- U01 DA041120/DA/NIDA NIH HHS/United States
- R01 DA047851/DA/NIDA NIH HHS/United States
- R01 NS124789/NS/NINDS NIH HHS/United States
- R01 HD088125/HD/NICHD NIH HHS/United States
- K99 MH129616/MH/NIMH NIH HHS/United States
- F32 MH120989/MH/NIMH NIH HHS/United States
- U01 NS108916/NS/NINDS NIH HHS/United States
- K23 NS123345/NS/NINDS NIH HHS/United States
- S10 MH124728/MH/NIMH NIH HHS/United States
- K99 MH121518/MH/NIMH NIH HHS/United States
- T32 DA007234/DA/NIDA NIH HHS/United States
- R01 MH116961/MH/NIMH NIH HHS/United States
- R01 MH120194/MH/NIMH NIH HHS/United States
- K12 NS098482/NS/NINDS NIH HHS/United States
- R01 MH121462/MH/NIMH NIH HHS/United States
- P30 DA048742/DA/NIDA NIH HHS/United States
- R01 MH118370/MH/NIMH NIH HHS/United States
- RF1 MH128696/MH/NIMH NIH HHS/United States
- R00 MH129616/MH/NIMH NIH HHS/United States
- R01 MH129426/MH/NIMH NIH HHS/United States
- P41 EB018783/EB/NIBIB NIH HHS/United States
- U01 DA041148/DA/NIDA NIH HHS/United States
- R01 EB031765/EB/NIBIB NIH HHS/United States
- R56 MH114976/MH/NIMH NIH HHS/United States
- R01 MH129493/MH/NIMH NIH HHS/United States
- R44 MH121276/MH/NIMH NIH HHS/United States
- R01 MH113883/MH/NIMH NIH HHS/United States
- R01 MH115357/MH/NIMH NIH HHS/United States
- F31 NS110332/NS/NINDS NIH HHS/United States
- R44 NS129521/NS/NINDS NIH HHS/United States
- P50 HD103525/HD/NICHD NIH HHS/United States
- R56 EB031765/EB/NIBIB NIH HHS/United States
- R44 MH124567/MH/NIMH NIH HHS/United States
- R44 MH122066/MH/NIMH NIH HHS/United States
- R01 HD055741/HD/NICHD NIH HHS/United States
- R00 MH121518/MH/NIMH NIH HHS/United States

