Human dendritic cell maturation induced by amorphous silica nanoparticles is Syk-dependent and triggered by lipid raft aggregation
- PMID: 37076877
- PMCID: PMC10114393
- DOI: 10.1186/s12989-023-00527-9
Human dendritic cell maturation induced by amorphous silica nanoparticles is Syk-dependent and triggered by lipid raft aggregation
Abstract
Background: Synthetic amorphous silica nanoparticles (SAS-NPs) are widely employed in pharmaceutics, cosmetics, food and concretes. Workers and the general population are exposed daily via diverse routes of exposure. SAS-NPs are generally recognized as safe (GRAS) by the Food and Drug Administration, but because of their nanoscale size and extensive uses, a better assessment of their immunotoxicity is required. In the presence of immune "danger signals", dendritic cells (DCs) undergo a maturation process resulting in their migration to regional lymph nodes where they activate naive T-cells. We have previously shown that fumed silica pyrogenic SAS-NPs promote the two first steps of the adaptative immune response by triggering DC maturation and T-lymphocyte response, suggesting that SAS-NPs could behave as immune "danger signals". The present work aims to identify the mechanism and the signalling pathways involved in DC phenotype modifications provoked by pyrogenic SAS-NPs. As a pivotal intracellular signalling molecule whose phosphorylation is associated with DC maturation, we hypothesized that Spleen tyrosine kinase (Syk) may play a central role in SAS-NPs-induced DC response.
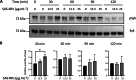
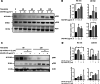
Results: In human monocyte-derived dendritic cells (moDCs) exposed to SAS-NPs, Syk inhibition prevented the induction of CD83 and CD86 marker expression. A significant decrease in T-cell proliferation and IFN-γ, IL-17F and IL-9 production was found in an allogeneic moDC:T-cell co-culture model. These results suggested that the activation of Syk was necessary for optimal co-stimulation of T-cells. Moreover, Syk phosphorylation, observed 30 min after SAS-NP exposure, occurred upstream of the c-Jun N-terminal kinase (JNK) Mitogen-activated protein kinases (MAPK) and was elicited by the Src family of protein tyrosine kinases. Our results also showed for the first time that SAS-NPs provoked aggregation of lipid rafts in moDCs and that MβCD-mediated raft destabilisation altered Syk activation.
Conclusions: We showed that SAS-NPs could act as an immune danger signal in DCs through a Syk-dependent pathway. Our findings revealed an original mechanism whereby the interaction of SAS-NPs with DC membranes promoted aggregation of lipid rafts, leading to a Src kinase-initiated activation loop triggering Syk activation and functional DC maturation.
Keywords: Amorphous silica; Dendritic cells; Lipid rafts; Nanoparticles; Src kinases; Syk.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
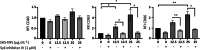
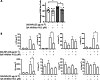




References
-
- Croissant JG, Butler KS, Zink JI, Brinker CJ. Synthetic amorphous silica nanoparticles: toxicity, biomedical and environmental implications. Nat Rev Mater. 2020;5(12):886–909. doi: 10.1038/s41578-020-0230-0. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

