Serum- and xeno-free culture of human umbilical cord perivascular cells for pediatric heart valve tissue engineering
- PMID: 37076906
- PMCID: PMC10116794
- DOI: 10.1186/s13287-023-03318-3
Serum- and xeno-free culture of human umbilical cord perivascular cells for pediatric heart valve tissue engineering
Abstract
Background: Constructs currently used to repair or replace congenitally diseased pediatric heart valves lack a viable cell population capable of functional adaptation in situ, necessitating repeated surgical intervention. Heart valve tissue engineering (HVTE) can address these limitations by producing functional living tissue in vitro that holds the potential for somatic growth and remodelling upon implantation. However, clinical translation of HVTE strategies requires an appropriate source of autologous cells that can be non-invasively harvested from mesenchymal stem cell (MSC)-rich tissues and cultured under serum- and xeno-free conditions. To this end, we evaluated human umbilical cord perivascular cells (hUCPVCs) as a promising cell source for in vitro production of engineered heart valve tissue.
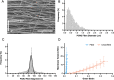
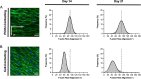
Methods: The proliferative, clonogenic, multilineage differentiation, and extracellular matrix (ECM) synthesis capacities of hUCPVCs were evaluated in a commercial serum- and xeno-free culture medium (StemMACS™) on tissue culture polystyrene and benchmarked to adult bone marrow-derived MSCs (BMMSCs). Additionally, the ECM synthesis potential of hUCPVCs was evaluated when cultured on polycarbonate polyurethane anisotropic electrospun scaffolds, a representative biomaterial for in vitro HVTE.
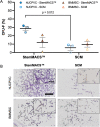
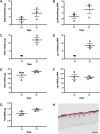
Results: hUCPVCs had greater proliferative and clonogenic potential than BMMSCs in StemMACS™ (p < 0.05), without differentiation to osteogenic and adipogenic phenotypes associated with valve pathology. Furthermore, hUCPVCs cultured with StemMACS™ on tissue culture plastic for 14 days synthesized significantly more total collagen, elastin, and sulphated glycosaminoglycans (p < 0.05), the ECM constituents of the native valve, than BMMSCs. Finally, hUCPVCs retained their ECM synthesizing capacity after 14 and 21 days in culture on anisotropic electrospun scaffolds.
Conclusion: Overall, our findings establish an in vitro culture platform that uses hUCPVCs as a readily-available and non-invasively sourced autologous cell population and a commercial serum- and xeno-free culture medium to increase the translational potential of future pediatric HVTE strategies. This study evaluated the proliferative, differentiation and extracellular matrix (ECM) synthesis capacities of human umbilical cord perivascular cells (hUCPVCs) when cultured in serum- and xeno-free media (SFM) against conventionally used bone marrow-derived MSCs (BMMSCs) and serum-containing media (SCM). Our findings support the use of hUCPVCs and SFM for in vitro heart valve tissue engineering (HVTE) of autologous pediatric valve tissue. Figure created with BioRender.com.
Keywords: Extracellular matrix; Heart valve tissue engineering; Human umbilical cord perivascular cells; Mesenchymal stromal cells; Serum- and xeno-free culture.
© 2023. The Author(s).
Conflict of interest statement
Dr. John E. Davies is the founding President and CEO of Tissue Regeneration Therapeutics, Inc. (Toronto, ON) who provided the human umbilical cord perivascular cells used in this study. The other authors declare no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

