Ginkgolide B facilitates muscle regeneration via rejuvenating osteocalcin-mediated bone-to-muscle modulation in aged mice
- PMID: 37076950
- PMCID: PMC10235878
- DOI: 10.1002/jcsm.13228
Ginkgolide B facilitates muscle regeneration via rejuvenating osteocalcin-mediated bone-to-muscle modulation in aged mice
Abstract
Background: The progressive deterioration of tissue-tissue crosstalk with aging causes a striking impairment of tissue homeostasis and functionality, particularly in the musculoskeletal system. Rejuvenation of the systemic and local milieu via interventions such as heterochronic parabiosis and exercise has been reported to improve musculoskeletal homeostasis in aged organisms. We have shown that Ginkgolide B (GB), a small molecule from Ginkgo biloba, improves bone homeostasis in aged mice by restoring local and systemic communication, implying a potential for maintaining skeletal muscle homeostasis and enhancing regeneration. In this study, we investigated the therapeutic efficacy of GB on skeletal muscle regeneration in aged mice.
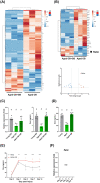
Methods: Muscle injury models were established by barium chloride induction into the hind limb of 20-month-old mice (aged mice) and into C2C12-derived myotubes. Therapeutic efficacy of daily administrated GB (12 mg/kg body weight) and osteocalcin (50 μg/kg body weight) on muscle regeneration was assessed by histochemical staining, gene expression, flow cytometry, ex vivo muscle function test and rotarod test. RNA sequencing was used to explore the mechanism of GB on muscle regeneration, with subsequent in vitro and in vivo experiments validating these findings.
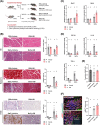
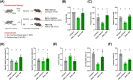
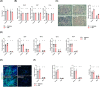
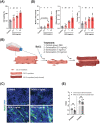
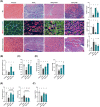
Results: GB administration in aged mice improved muscle regeneration (muscle mass, P = 0.0374; myofiber number/field, P = 0.0001; centre nucleus, embryonic myosin heavy chain-positive myofiber area, P = 0.0144), facilitated the recovery of muscle contractile properties (tetanic force, P = 0.0002; twitch force, P = 0.0005) and exercise performance (rotarod performance, P = 0.002), and reduced muscular fibrosis (collagen deposition, P < 0.0001) and inflammation (macrophage infiltration, P = 0.03). GB reversed the aging-related decrease in the expression of osteocalcin (P < 0.0001), an osteoblast-specific hormone, to promote muscle regeneration. Exogenous osteocalcin supplementation was sufficient to improve muscle regeneration (muscle mass, P = 0.0029; myofiber number/field, P < 0.0001), functional recovery (tetanic force, P = 0.0059; twitch force, P = 0.07; rotarod performance, P < 0.0001) and fibrosis (collagen deposition, P = 0.0316) in aged mice, without an increased risk of heterotopic ossification.
Conclusions: GB treatment restored the bone-to-muscle endocrine axis to reverse aging-related declines in muscle regeneration and thus represents an innovative and practicable approach to managing muscle injuries. Our results revealed the critical and novel role of osteocalcin-GPRC6A-mediated bone-to-muscle communication in muscle regeneration, which provides a promising therapeutic avenue in functional muscle regeneration.
Keywords: Ginkgolide B; aging; osteocalcin; rejuvenation; skeletal muscle regeneration.
© 2023 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005;433:760–764. - PubMed
-
- Tagliaferri C, Wittrant Y, Davicco MJ, Walrand S, Coxam V. Muscle and bone, two interconnected tissues. Ageing Res Rev 2015;21:55–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

