Development of polarization-sensitive optical coherence tomography imaging platform and metrics to quantify electrostimulation-induced peripheral nerve injury in vivo in a small animal model
- PMID: 37077218
- PMCID: PMC10109528
- DOI: 10.1117/1.NPh.10.2.025004
Development of polarization-sensitive optical coherence tomography imaging platform and metrics to quantify electrostimulation-induced peripheral nerve injury in vivo in a small animal model
Abstract
Significance: Neuromodulation devices are rapidly evolving for the treatment of neurological diseases and conditions. Injury from implantation or long-term use without obvious functional losses is often only detectable through terminal histology. New technologies are needed that assess the peripheral nervous system (PNS) under normal and diseased or injured conditions.
Aim: We aim to demonstrate an imaging and stimulation platform that can elucidate the biological mechanisms and impacts of neurostimulation in the PNS and apply it to the sciatic nerve to extract imaging metrics indicating electrical overstimulation.
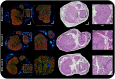
Approach: A sciatic nerve injury model in a 15-rat cohort was observed using a newly developed imaging and stimulation platform that can detect electrical overstimulation effects with polarization-sensitive optical coherence tomography. The sciatic nerve was electrically stimulated using a custom-developed nerve holder with embedded electrodes for 1 h, followed by a 1-h recovery period, delivered at above-threshold Shannon model -values in experimental groups: sham control (SC, , ), stimulation level 1 (SL1, , , and ), and stimulation level 2 (SL2, , , and ).
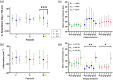
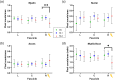
Results: The stimulation and imaging system successfully captured study data across the cohort. When compared to a SC after a 1-week recovery, the fascicle closest to the stimulation lead showed an average change of (SL1/SL2) in phase retardation and in optical attenuation relative to SC. Analysis of immunohistochemistry (IHC) shows a difference in myelin pixel counts and difference in axon pixel counts, and an overall increase in cell nuclei pixel count of . These metrics were consistent with IHC and hematoxylin/eosin tissue section analysis.
Conclusions: The poststimulation changes observed in our study are manifestations of nerve injury and repair, specifically degeneration and angiogenesis. Optical imaging metrics quantify these processes and may help evaluate the safety and efficacy of neuromodulation devices.
Keywords: nerve stimulation; neuromodulation; optical metrics; peripheral nerve injury; polarization-sensitive optical coherence tomography; rat; sciatic nerve.
© 2023 The Authors.
Figures





Similar articles
-
Label-free full-thickness imaging of porcine vagus nerve fascicular anatomy by polarization-sensitive optical coherence tomography.J Neural Eng. 2025 Feb 28;22(2):10.1088/1741-2552/adb5c3. doi: 10.1088/1741-2552/adb5c3. J Neural Eng. 2025. PMID: 39946850
-
Toward optical coherence tomography angiography-based biomarkers to assess the safety of peripheral nerve electrostimulation.J Neural Eng. 2019 Jun;16(3):036024. doi: 10.1088/1741-2552/ab1405. Epub 2019 Mar 27. J Neural Eng. 2019. PMID: 30917357 Free PMC article.
-
An experimental model of an electrical injury to the peripheral nerve.Burns. 2005 Sep;31(6):731-6. doi: 10.1016/j.burns.2005.02.022. Epub 2005 Apr 25. Burns. 2005. PMID: 16129227
-
Down-regulation miR-146a-5p in Schwann cell-derived exosomes induced macrophage M1 polarization by impairing the inhibition on TRAF6/NF-κB pathway after peripheral nerve injury.Exp Neurol. 2023 Apr;362:114295. doi: 10.1016/j.expneurol.2022.114295. Epub 2022 Dec 6. Exp Neurol. 2023. PMID: 36493861
-
Effect of the combination of high-frequency repetitive magnetic stimulation and neurotropin on injured sciatic nerve regeneration in rats.Neural Regen Res. 2020 Jan;15(1):145-151. doi: 10.4103/1673-5374.264461. Neural Regen Res. 2020. PMID: 31535663 Free PMC article.
Cited by
-
Label-free full-thickness imaging of porcine vagus nerve fascicular anatomy by polarization-sensitive optical coherence tomography.J Neural Eng. 2025 Feb 28;22(2):10.1088/1741-2552/adb5c3. doi: 10.1088/1741-2552/adb5c3. J Neural Eng. 2025. PMID: 39946850
-
Neurosurgical Intervention for Nerve and Muscle Biopsies.Diagnostics (Basel). 2024 May 31;14(11):1169. doi: 10.3390/diagnostics14111169. Diagnostics (Basel). 2024. PMID: 38893695 Free PMC article. Review.

