Free-aldehyde neutralized and oligohyaluronan loaded bovine pericardium with improved anti-calcification and endothelialization for bioprosthetic heart valves
- PMID: 37077226
- PMCID: PMC10106738
- DOI: 10.3389/fbioe.2023.1138972
Free-aldehyde neutralized and oligohyaluronan loaded bovine pericardium with improved anti-calcification and endothelialization for bioprosthetic heart valves
Abstract
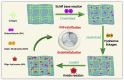
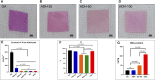
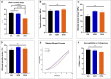
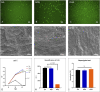
The number of patients with valvular heart disease is increasing yearly, and valve replacement is the most effective treatment, during which bioprosthetic heart valves (BHVs) are the most widely used. Commercial BHVs are mainly prepared with glutaraldehyde (Glut) cross-linked bovine pericardial or porcine aortic valves, but the residual free aldehyde groups in these tissues can cause calcification and cytotoxicity. Moreover, insufficient glycosaminoglycans (GAGs) in tissues can further reduce biocompatibility and durability. However, the anti-calcification performance and biocompatibility might be improved by blocking the free aldehyde groups and increasing the GAGs content in Glut-crosslinked tissues. In our study, adipic dihydrazide (ADH) was used to neutralize the residual free aldehyde groups in tissues and provide sites to blind with oligohyaluronan (OHA) to increase the content of GAGs in tissues. The modified bovine pericardium was evaluated for its content of residual aldehyde groups, the amount of OHA loaded, physical/chemical characteristics, biomechanical properties, biocompatibility, and in vivo anticalcification assay and endothelialization effects in juvenile Sprague-Dawley rats. The results showed that ADH could completely neutralize the free aldehyde groups in the Glut-crosslinked bovine pericardium, the amount of OHA loaded increased and the cytotoxicity was reduced. Moreover, the in vivo results also showed that the level of calcification and inflammatory response in the modified pericardial tissue was significantly reduced in a rat subcutaneous implantation model, and the results from the rat abdominal aorta vascular patch repair model further demonstrated the improved capability of the modified pericardial tissues for endothelialization. Furthermore, more α-SMA+ smooth muscle cells and fewer CD68+ macrophages infiltrated in the neointima of the modified pericardial patch. In summary, blocking free-aldehydes and loading OHA improved the anti-calcification, anti-inflammation and endothelialization properties of Glut-crosslinked BHVs and in particularly, this modified strategy may be a promising candidate for the next-generation of BHVs.
Keywords: adipic dihydrazides; anti-calcification; bioprosthetic heart valves; endothelialization; oligo-hyaluronan.
Copyright © 2023 Liu, Chen, Lu, Liu, Wu and Tang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Bramsen J. A., Alber B. R., Mendoza M., Murray B. T., Chen M. H., Huang P., et al. (2022). Glycosaminoglycans affect endothelial to mesenchymal transformation, proliferation, and calcification in a 3D model of aortic valve disease. Front. Cardiovasc Med. 9, 975732. 10.3389/fcvm.2022.975732 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

