Identification of fish spermatogenic cells through high-throughput immunofluorescence against testis with an antibody set
- PMID: 37077350
- PMCID: PMC10106697
- DOI: 10.3389/fendo.2023.1044318
Identification of fish spermatogenic cells through high-throughput immunofluorescence against testis with an antibody set
Abstract
Image-based identification and quantification of different types of spermatogenic cells is of great importance, not only for reproductive studies but also for genetic breeding. Here, we have developed antibodies against spermatogenesis-related proteins in zebrafish (Danio rerio), including Ddx4, Piwil1, Sycp3, and Pcna, and a high-throughput method for immunofluorescence analysis of zebrafish testicular sections. By immunofluorescence analysis of zebrafish testes, our results demonstrate that the expression of Ddx4 decreases progressively during spermatogenesis, Piwil1 is strongly expressed in type A spermatogonia and moderately expressed in type B spermatogonia, and Sycp3 has distinct expression patterns in different subtypes of spermatocytes. Additionally, we observed polar expression of Sycp3 and Pcna in primary spermatocytes at the leptotene stage. By a triple staining of Ddx4, Sycp3, and Pcna, different types/subtypes of spermatogenic cells were easily characterized. We further demonstrated the practicality of our antibodies in other fish species, including Chinese rare minnow (Gobiocypris rarus), common carp (Cyprinus carpio), blunt snout bream (Megalobrama amblycephala), rice field eel (Monopterus albus) and grass carp (Ctenopharyngodon idella). Finally, we proposed an integrated criterion for identifying different types/subtypes of spermatogenic cells in zebrafish and other fishes using this high-throughput immunofluorescence approach based on these antibodies. Therefore, our study provides a simple, practical, and efficient tool for the study of spermatogenesis in fish species.
Keywords: Ddx4; PCNA; Piwil1; Sycp3; spermatocyte; spermatogonia.
Copyright © 2023 Ye, Liu, Li, Wang, Hu, Zhu and Sun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


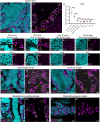
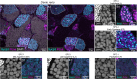


Similar articles
-
Surface location and stage-specificity of differentiation antigens on germ cells in the common carp (Cyprinus carpio), as revealed with monoclonal antibodies and immunogold staining.Histochemistry. 1990;95(1):77-85. doi: 10.1007/BF00737231. Histochemistry. 1990. PMID: 2286535
-
Evaluation of Sycp3, Plzf and Cyclin B3 expression and suitability as spermatogonia and spermatocyte markers in zebrafish.Gene Expr Patterns. 2011 Jun-Jul;11(5-6):309-15. doi: 10.1016/j.gep.2011.03.002. Epub 2011 Mar 21. Gene Expr Patterns. 2011. PMID: 21402175
-
Dynamics, ultrastructure and gene expression of human in vitro organized testis cells from testicular sperm extraction biopsies.Mol Hum Reprod. 2018 Mar 1;24(3):123-134. doi: 10.1093/molehr/gax070. Mol Hum Reprod. 2018. PMID: 29304256
-
The protective effects of selenium-enriched spirulina on the reproductive system of male zebrafish (Danio rerio) exposed to beta-cypermethrin.Food Funct. 2018 Nov 14;9(11):5791-5804. doi: 10.1039/c8fo01527a. Food Funct. 2018. PMID: 30352112
-
Spermatogonial proliferation patterns in men with azoospermia of different etiologies.Fertil Steril. 2003 Nov;80(5):1175-80. doi: 10.1016/s0015-0282(03)02161-7. Fertil Steril. 2003. PMID: 14607570
Cited by
-
foxl2l is a germ cell-intrinsic gatekeeper of oogenesis in zebrafish.Zool Res. 2024 Sep 18;45(5):1116-1130. doi: 10.24272/j.issn.2095-8137.2024.046. Zool Res. 2024. PMID: 39257375 Free PMC article.
-
Induced formation of primordial germ cells from zebrafish blastomeres by germplasm factors.Nat Commun. 2023 Dec 14;14(1):7918. doi: 10.1038/s41467-023-43587-3. Nat Commun. 2023. PMID: 38097571 Free PMC article.
-
Establishment of a Coilia nasus Spermatogonial Stem Cell Line Capable of Spermatogenesis In Vitro.Biology (Basel). 2023 Aug 28;12(9):1175. doi: 10.3390/biology12091175. Biology (Basel). 2023. PMID: 37759575 Free PMC article.
References
-
- Gui J, Zhou L, Li X. Rethinking fish biology and biotechnologies in the challenge era for burgeoning genome resources and strengthening food security. Water Biol Secur (2022) 1(1):100002. doi: 10.1016/j.watbs.2021.11.001 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

