Discovery and Optimization of a Novel Macrocyclic Amidinourea Series Active as Acidic Mammalian Chitinase Inhibitors
- PMID: 37077400
- PMCID: PMC10107916
- DOI: 10.1021/acsmedchemlett.2c00472
Discovery and Optimization of a Novel Macrocyclic Amidinourea Series Active as Acidic Mammalian Chitinase Inhibitors
Abstract
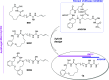
Our research group has been involved for a long time in the development of macrocyclic amidinoureas (MCAs) as antifungal agents. The mechanistic investigation drove us to perform an in silico target fishing study, which allowed the identification of chitinases as one of their putative targets, with 1a showing a submicromolar inhibition of Trichoderma viride chitinase. In this work, we investigated the possibility to further inhibit the corresponding human enzymes, acidic mammalian chitinase (AMCase) and chitotriosidase (CHIT1), involved in several chronic inflammatory lung diseases. Thus, we first validated the inhibitory activity of 1a against AMCase and CHIT1 and then designed and synthesized new derivatives aimed at improving the potency and selectivity against AMCase. Among them, compound 3f emerged for its activity profile along with its promising in vitro ADME properties. We also gained a good understanding of the key interactions with the target enzyme through in silico studies.
© 2023 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

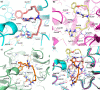
Similar articles
-
Direct comparison of chitinolytic properties and determination of combinatory effects of mouse chitotriosidase and acidic mammalian chitinase.Int J Biol Macromol. 2019 Aug 1;134:882-890. doi: 10.1016/j.ijbiomac.2019.05.097. Epub 2019 May 17. Int J Biol Macromol. 2019. PMID: 31108147
-
Design and synthesis of a novel inhibitor of T. Viride chitinase through an in silico target fishing protocol.Bioorg Med Chem Lett. 2017 Aug 1;27(15):3332-3336. doi: 10.1016/j.bmcl.2017.06.016. Epub 2017 Jun 10. Bioorg Med Chem Lett. 2017. PMID: 28610983
-
Chitotriosidase is the primary active chitinase in the human lung and is modulated by genotype and smoking habit.J Allergy Clin Immunol. 2008 Nov;122(5):944-950.e3. doi: 10.1016/j.jaci.2008.08.023. Epub 2008 Oct 9. J Allergy Clin Immunol. 2008. PMID: 18845328 Free PMC article.
-
Human Chitinases: Structure, Function, and Inhibitor Discovery.Adv Exp Med Biol. 2019;1142:221-251. doi: 10.1007/978-981-13-7318-3_11. Adv Exp Med Biol. 2019. PMID: 31102249 Review.
-
Chitinases and Chitinase-Like Proteins as Therapeutic Targets in Inflammatory Diseases, with a Special Focus on Inflammatory Bowel Diseases.Int J Mol Sci. 2021 Jun 28;22(13):6966. doi: 10.3390/ijms22136966. Int J Mol Sci. 2021. PMID: 34203467 Free PMC article. Review.
Cited by
-
Human chitinases and chitinase-like proteins as emerging drug targets - a medicinal chemistry perspective.RSC Med Chem. 2025 Apr 24;16(6):2388-2402. doi: 10.1039/d4md01050g. eCollection 2025 Jun 18. RSC Med Chem. 2025. PMID: 40313579 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Chemical Information
Miscellaneous

