ChemBeads-Enabled Photoredox High-Throughput Experimentation Platform to Improve C(sp2)-C(sp3) Decarboxylative Couplings
- PMID: 37077401
- PMCID: PMC10108395
- DOI: 10.1021/acsmedchemlett.2c00538
ChemBeads-Enabled Photoredox High-Throughput Experimentation Platform to Improve C(sp2)-C(sp3) Decarboxylative Couplings
Abstract
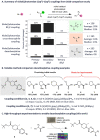
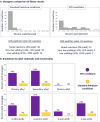
Enthusiasm surrounding nickel/photoredox C(sp2)-C(sp3) cross-couplings is very high; however, these methods are sometimes challenged by complex drug-like substrates in discovery chemistry. In our hands this has been especially true of the decarboxylative coupling, which has lagged behind other photoredox couplings in internal adoption and success. Herein, the development of a photoredox high-throughput experimentation platform to optimize challenging C(sp2)-C(sp3) decarboxylative couplings is described. Chemical-coated glass beads (ChemBeads) and a novel parallel bead dispenser are used to expedite the high-throughput experimentation process and identify improved coupling conditions. In this report, photoredox high-throughput experimentation is utilized to dramatically improve low-yielding decarboxylative C(sp2)-C(sp3) couplings, and libraries, using conditions not previously identified in the literature.
© 2023 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Dual catalysis. Merging photoredox with nickel catalysis: coupling of α-carboxyl sp³-carbons with aryl halides.Science. 2014 Jul 25;345(6195):437-40. doi: 10.1126/science.1255525. Epub 2014 Jun 5. Science. 2014. PMID: 24903563 Free PMC article.
-
Visible Light Activation of Boronic Esters Enables Efficient Photoredox C(sp2 )-C(sp3 ) Cross-Couplings in Flow.Angew Chem Int Ed Engl. 2016 Nov 2;55(45):14085-14089. doi: 10.1002/anie.201605548. Epub 2016 Oct 6. Angew Chem Int Ed Engl. 2016. PMID: 27709749 Free PMC article.
-
Single-Electron Transmetalation via Photoredox/Nickel Dual Catalysis: Unlocking a New Paradigm for sp(3)-sp(2) Cross-Coupling.Acc Chem Res. 2016 Jul 19;49(7):1429-39. doi: 10.1021/acs.accounts.6b00214. Epub 2016 Jul 5. Acc Chem Res. 2016. PMID: 27379472 Free PMC article.
-
At the Speed of Light: The Systematic Implementation of Photoredox Cross-Coupling Reactions for Medicinal Chemistry Research.J Org Chem. 2024 Nov 15;89(22):16070-16092. doi: 10.1021/acs.joc.3c02351. Epub 2024 Mar 5. J Org Chem. 2024. PMID: 38442262 Review.
-
Photoredox-Mediated Routes to Radicals: The Value of Catalytic Radical Generation in Synthetic Methods Development.ACS Catal. 2017 Apr 7;7(4):2563-2575. doi: 10.1021/acscatal.7b00094. Epub 2017 Mar 14. ACS Catal. 2017. PMID: 28413692 Free PMC article. Review.
Cited by
-
Predictions of Chromatography Methods by Chemical Structure Similarity to Accelerate High-Throughput Medicinal Chemistry.ACS Med Chem Lett. 2024 Jul 12;15(8):1396-1401. doi: 10.1021/acsmedchemlett.4c00145. eCollection 2024 Aug 8. ACS Med Chem Lett. 2024. PMID: 39140053 Free PMC article.
-
A Miniaturized Chemical High Throughput Experimentation Plate to Enable Late-Stage Oxidation Screening and Scale-up.ACS Med Chem Lett. 2025 Jun 2;16(6):916-924. doi: 10.1021/acsmedchemlett.5c00175. eCollection 2025 Jun 12. ACS Med Chem Lett. 2025. PMID: 40529082
-
Enabling synthesis in fragment-based drug discovery (FBDD): microscale high-throughput optimisation of the medicinal chemist's toolbox reactions.RSC Med Chem. 2023 Nov 24;14(12):2699-2713. doi: 10.1039/d3md00495c. eCollection 2023 Dec 13. RSC Med Chem. 2023. PMID: 38107176 Free PMC article.
-
Light-assisted carbon dioxide reduction in an automated photoreactor system coupled to carbonylation chemistry.Chem Sci. 2024 Nov 8;15(47):19842-19850. doi: 10.1039/d4sc06660j. eCollection 2024 Dec 4. Chem Sci. 2024. PMID: 39568953 Free PMC article.
-
An Air-Stable, Single-Component Iridium Precatalyst for the Borylation of C-H Bonds on Large to Miniaturized Scales.J Am Chem Soc. 2024 Nov 27;146(47):32717-32729. doi: 10.1021/jacs.4c12333. Epub 2024 Nov 12. J Am Chem Soc. 2024. PMID: 39531608
References
-
- Cernak T.; Gesmundo N. J.; Dykstra K.; Yu Y.; Wu Z.; Shi Z.-C.; Vachal P.; Sperbeck D.; He S.; Murphy B. A.; Sonatore L.; Williams S.; Madeira M.; Verras A.; Reiter M.; Lee C. H.; Cuff J.; Sherer E. C.; Kuethe J.; Goble S.; Perrotto N.; Pinto S.; Shen D.-M.; Nargund R.; Balkovec J.; DeVita R. J.; Dreher S. D. Microscale High-Throughput Experimentation as an Enabling Technology in Drug Discovery: Application in the Discovery of (Piperidinyl)Pyridinyl-1H-Benzimidazole Diacylglycerol Acyltransferase 1 Inhibitors. J. Med. Chem. 2017, 60 (9), 3594–3605. 10.1021/acs.jmedchem.6b01543. - DOI - PubMed
-
- Santanilla A. B.; Regalado E. L.; Pereira T.; Shevlin M.; Bateman K.; Campeau L.-C.; Schneeweis J.; Berritt S.; Shi Z.-C.; Nantermet P.; Liu Y.; Helmy R.; Welch C. J.; Vachal P.; Davies I. W.; Cernak T.; Dreher S. D. Nanomole-Scale High-Throughput Chemistry for the Synthesis of Complex Molecules. Science 2015, 347 (6217), 49–53. 10.1126/science.1259203. - DOI - PubMed
LinkOut - more resources
Full Text Sources

