Phytochemical component and toxicological evaluation of purple sweet potato leaf extract in male Sprague-Dawley rats
- PMID: 37077809
- PMCID: PMC10106777
- DOI: 10.3389/fphar.2023.1132087
Phytochemical component and toxicological evaluation of purple sweet potato leaf extract in male Sprague-Dawley rats
Abstract
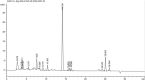
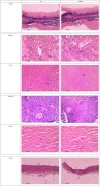
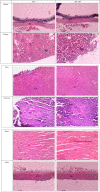
This study assessed the toxicity of lutein-rich purple sweet potato leaf (PSPL) extract in male Sprague-Dawley rats. Methods and study design: A total of 54 adult male Sprague-Dawley rats were used. For the acute toxicity study, three rats in the acute control group were fed 2,000 mg/kg of PSPL for 14 days. The subacute toxicity study included six rats each in four groups administered 50, 250, 500, or 1,000 mg/kg for 28 days and observed for further 14 days without treatment in the subacute control and subacute satellite groups. Changes in body weight; blood biochemistry; hematological parameters; relative organ weight; and histological sections of the heart, kidney, liver, pancreas, aorta, and retina were observed for signs of toxicity. Results: The gradual increase in weekly body weight, normal level full blood count, normal liver and kidney profile, relative organ weight, and histological sections of all stained organ tissue in the treated group compared with the acute, subacute, and satellite control groups demonstrated the absence of signs of toxicity. Conclusion: Lutein-rich PSPL extract shows no signs of toxicity up to 2,000 mg/kg/day.
Keywords: animal study; bioactive compounds; medicinal plant; phytochemicals; plant extract; purple sweet potato; toxicity.
Copyright © 2023 Hisamuddin, Naomi, Bin Manan, Bahari, Yazid, Othman, Embong, Hadizah Jumidil, Hussain and Zakaria.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
The role of lutein-rich purple sweet potato leaf extract on the amelioration of diabetic retinopathy in streptozotocin-induced Sprague-Dawley rats.Front Pharmacol. 2023 May 18;14:1175907. doi: 10.3389/fphar.2023.1175907. eCollection 2023. Front Pharmacol. 2023. PMID: 37274105 Free PMC article.
-
Acute and sub-chronic oral toxicity study of purple sweet potato (Ipomoea batatas [L.] Lam) yogurt in mice (Mus musculus).Vet World. 2022 Mar;15(3):789-796. doi: 10.14202/vetworld.2022.789-796. Epub 2022 Mar 31. Vet World. 2022. PMID: 35497941 Free PMC article.
-
Bioactive chemical constituents, in vitro anti-proliferative activity and in vivo toxicity of the extract of Curcuma singularis Gagnep rhizomes.J Ethnopharmacol. 2022 Feb 10;284:114803. doi: 10.1016/j.jep.2021.114803. Epub 2021 Nov 6. J Ethnopharmacol. 2022. PMID: 34748866
-
Phytochemical Analysis and Toxicity Assessment of Bouea Macrophylla Yoghurt.Toxins (Basel). 2023 Feb 3;15(2):125. doi: 10.3390/toxins15020125. Toxins (Basel). 2023. PMID: 36828439 Free PMC article.
-
Evaluation of acute and subacute toxicity of whole-plant aqueous extract of Vernonia mespilifolia Less. in Wistar rats.J Integr Med. 2018 Sep;16(5):335-341. doi: 10.1016/j.joim.2018.07.003. Epub 2018 Jul 4. J Integr Med. 2018. PMID: 30007829
Cited by
-
Safety assessment of Acori Tatarinowii Rhizoma: acute and subacute oral toxicity.Front Pharmacol. 2024 Mar 19;15:1377876. doi: 10.3389/fphar.2024.1377876. eCollection 2024. Front Pharmacol. 2024. PMID: 38567357 Free PMC article.
-
The role of lutein-rich purple sweet potato leaf extract on the amelioration of diabetic retinopathy in streptozotocin-induced Sprague-Dawley rats.Front Pharmacol. 2023 May 18;14:1175907. doi: 10.3389/fphar.2023.1175907. eCollection 2023. Front Pharmacol. 2023. PMID: 37274105 Free PMC article.
-
Reclaiming Agriceuticals from Sweetpotato (Ipomoea batatas [L.] Lam.) By-Products.Foods. 2024 Apr 12;13(8):1180. doi: 10.3390/foods13081180. Foods. 2024. PMID: 38672853 Free PMC article. Review.
References
-
- Alam M. K. (2021). A comprehensive review of sweet potato (Ipomoea batatas [L.] Lam): Revisiting the associated health benefits. Trends Food Sci. Technol. 115, 512–529. 10.1016/J.TIFS.2021.07.001 - DOI
-
- Bahari H., Abidin A. Z., Balan S. S., Perumal K. V., Rosli N. S., Lotafi A. H. A., et al. (2020). The effects of Elateriospermum tapos against obese maternal rat in mitigating obesity development among their adult female offspring. Pharmacogn. Mag. 16, 706–712. 10.4103/pm.pm_142_20 - DOI
-
- Balan S. S., Abidin A. Z., Perumal K. V., Shafie N. H., Abdullah M. A., Jasni A. S., et al. (2021). Transgenerational evaluation of Elateriospermum tapos extracts on the male offspring of obesity-induced sprague dawley rats. Sains Malays. 50, 3045–3057. 10.17576/jsm-2021-5010-17 - DOI
LinkOut - more resources
Full Text Sources

