The utility of 3D models to study cholesterol in cancer: Insights and future perspectives
- PMID: 37077827
- PMCID: PMC10106729
- DOI: 10.3389/fonc.2023.1156246
The utility of 3D models to study cholesterol in cancer: Insights and future perspectives
Abstract
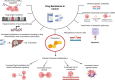
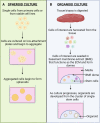
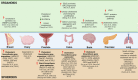
Cholesterol remains a vital molecule required for life; however, increasing evidence exists implicating cholesterol in cancer development and progression. Numerous studies investigating the relationship between cholesterol and cancer in 2-dimensional (2D) culture settings exist, however these models display inherent limitations highlighting the incipient need to develop better models to study disease pathogenesis. Due to the multifaceted role cholesterol plays in the cell, researchers have begun utilizing 3-dimensional (3D) culture systems, namely, spheroids and organoids to recapitulate cellular architecture and function. This review aims to describe current studies exploring the relationship between cancer and cholesterol in a variety of cancer types using 3D culture systems. We briefly discuss cholesterol dyshomeostasis in cancer and introduce 3D in-vitro culture systems. Following this, we discuss studies performed in cancerous spheroid and organoid models that focused on cholesterol, highlighting the dynamic role cholesterol plays in various cancer types. Finally, we attempt to provide potential gaps in research that should be explored in this rapidly evolving field of study.
Keywords: 3D culture; cancer; cholesterol; organoids; spheroids.
Copyright © 2023 du Plessis, Abdulla and Kaur.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Hanahan D, Weinberg RA. Biological hallmarks of cancer. Holland-Frei Cancer Med (2017) 2008. doi: 10.1002/9781119000822.hfcm002 - DOI
-
- Hanahan D. Hallmarks of cancer: New dimensions. Cancer Discovery (2022) 12(1):31–46. doi: 10.1158/2159-8290.CD-21-1059 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

