Real-life data of abiraterone acetate and enzalutamide treatment in post-chemotherapy metastatic castration-resistant prostate cancer in Poland
- PMID: 37077831
- PMCID: PMC10108911
- DOI: 10.3389/fonc.2023.1108937
Real-life data of abiraterone acetate and enzalutamide treatment in post-chemotherapy metastatic castration-resistant prostate cancer in Poland
Abstract
Background: Abiraterone acetate (ABI) and Enzalutamide (ENZA) are second-generation hormone drugs that show breakthrough activity in post-chemotherapy, metastatic castration-resistant prostate cancer (mCRPC). The leading oncological and urological guidelines indicate both drugs with the same strong recommendation. There is a lack of randomized trials which compare the efficacy of ABI and ENZA. The current study aimed to compare the effectiveness of the drugs with an analysis of prognostic factors related to those drugs.
Patients and methods: The study included 420 patients with docetaxel (DXL) pretreated mCRPC from seven Polish cancer centers. Patients were treated according to inclusion and exclusion criteria in the Polish national drug program (1000 mg ABI and 10 mg prednisone, n=76.2%; ENZA, 160 mg; n=23.8%). The study retrospectively analyzed the overall survival (OS), time to treatment failure (TTF), PSA 50% decline rate (PSA 50%) and selected clinic-pathological data.
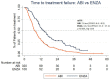
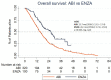
Results: In the study group, the median OS was 17 months (95% CI: 15.6-18.3). The median OS (26.1 vs. 15.7 mo.; p<0.001), TTF (14.2 vs. 7.6 mo.; p<0.001) and PSA 50% (87.5 vs. 56%; p<0.001) were higher in ENZA than in ABI treatment. Multivariate analysis shows that ENZA treatment and PSA nadir <17.35 ng/mL during or after DXL treatment were related to longer TTF. ENZA treatment, DXL dose ≥750 mg, PSA nadir <17.35 ng/mL during or after DXL treatment was related to longer OS.
Conclusions: ENZA treatment may be related to more favorable oncological outcomes than ABI treatment in the studied Polish population of patients. A 50% decline in PSA is an indicator of longer TTF and OS. Due to the non-randomized and retrospective nature of the analysis, the current results require prospective validation.
Keywords: abiraterone acetate; enzalutamide; metastatic prostate cancer; real-word study; targeted therapy.
Copyright © 2023 Sigorski, Wilk, Gawlik-Urban, Sałek-Zań, Kiszka, Malik, Czerko, Kuć, Szczylik, Kubiatowski, Cybulska-Stopa, Filipczyk-Cisarż, Bodnar and Skoneczna.
Conflict of interest statement
DS and KK received honoraria for lectures from Astellas and Janssen. MW received travel grants from Pfizer, Novartis and Bayer, and honoraria for lectures from Pfizer. KC received travel grants from Janssen. IS received grants/research support, honoraria or consultation fees from Astellas, Janssen. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Surveillance, epidemiology, and end results program. Available at: https://seer.cancer.gov/ (Accessed July 28, 2022).
-
- Freedman-Cass D, Berardi R, Shead DA, Schaeffer EM, An Y, Armstrong AJ, et al. . NCCN guidelines version 4.2022 prostate cancer. Available at: https://www.nccn.org/home/ (Accessed July 28, 2022).
-
- Cornford P, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. . EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. part II–2020 update: Treatment of relapsing and metastatic prostate Cancer[Formula presented]. Eur Urol (2021) 79:263–82. doi: 10.1016/J.EURURO.2020.09.046 - DOI - PubMed
-
- Potocki LP, Potocki PM, Wysocki PJ. Evolution of prostate cancer therapy. part 1. Oncol Clin Pract (2022) 18:177–88. doi: 10.5603/OCP.2021.0001 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous