Clinical impacts of the concomitant use of L-asparaginase and total parenteral nutrition containing L-aspartic acid in patients with acute lymphoblastic leukemia
- PMID: 37077904
- PMCID: PMC10106764
- DOI: 10.3389/fnut.2023.1122010
Clinical impacts of the concomitant use of L-asparaginase and total parenteral nutrition containing L-aspartic acid in patients with acute lymphoblastic leukemia
Abstract
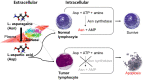
Introduction: L-asparaginase (ASNase) depletes L-asparagine and causes the death of leukemic cells, making it a mainstay for the treatment of acute lymphoblastic leukemia (ALL). However, ASNase's activity can be inhibited by L-aspartic acid (Asp), which competes for the same substrate and reduces the drug's efficacy. While many commercially used total parenteral nutrition (TPN) products contain Asp, it is unclear how the concomitant use of TPNs containing Asp (Asp-TPN) affects ALL patients treated with ASNase. This propensity-matched retrospective cohort study evaluated the clinical effects of the interaction between ASNase and Asp-TPN.
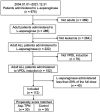
Methods: The study population included newly diagnosed adult Korean ALL patients who received VPDL induction therapy consisting of vincristine, prednisolone, daunorubicin, and Escherichia coli L-asparaginase between 2004 and 2021. Patients were divided into two groups based on their exposure to Asp-TPN: (1) Asp-TPN group and (2) control group. Data, including baseline characteristics, disease information, medication information, and laboratory data, were collected retrospectively. The primary outcomes for the effectiveness were overall and complete response rates. Relapse-free survival at six months and one year of treatment were also evaluated. The safety of both TPN and ASNase was evaluated by comparing liver function test levels between groups. A 1:1 propensity score matching analysis was conducted to minimize potential selection bias.
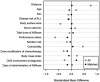
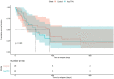
Results: The analysis included a total of 112 ALL patients, and 34 of whom received Asp-TPN and ASNase concomitantly. After propensity score matching, 30 patients remained in each group. The concomitant use of Asp-TPN and ASNase did not affect the overall response rate (odds ratio [OR] 0.53; 95% confidence interval [CI] = 0.17-1.62) or the complete response rate (OR 0.86; 95% CI = 0.29-2.59) of the ASNase-including induction therapy. The concomitant use of Asp-TPN and ASNase also did not impact relapse-free survival (RFS) at six months and one year of treatment (OR 1.00; 95% CI = 0.36-2.78 and OR 1.24; 95% CI, 0.50-3.12, respectively). The peak levels of each liver function test (LFT) and the frequency of LFT elevations were evaluated during induction therapy and showed no difference between the two groups.
Conclusion: There is no clear rationale for avoiding Asp-TPN in ASNase-treated patients.
Keywords: L-asparaginase; L-aspartic acid; acute lymhoblastic leukaemia; effectiveness; interaction; propensity score matching; safety; total parenteral nutrition.
Copyright © 2023 Ko, Kim, Yoon, Kim, Suh, Cho and Oh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Assessment of l-Asparaginase Pharmacodynamics in Mouse Models of Cancer.Metabolites. 2019 Jan 9;9(1):10. doi: 10.3390/metabo9010010. Metabolites. 2019. PMID: 30634463 Free PMC article.
-
Asparaginase pharmacokinetics after intensive polyethylene glycol-conjugated L-asparaginase therapy for children with relapsed acute lymphoblastic leukemia.Clin Cancer Res. 2004 Aug 15;10(16):5335-41. doi: 10.1158/1078-0432.CCR-04-0222. Clin Cancer Res. 2004. PMID: 15328169
-
Comparison of intramuscular therapy with Erwinia asparaginase and asparaginase Medac: pharmacokinetics, pharmacodynamics, formation of antibodies and influence on the coagulation system.Br J Haematol. 2001 Dec;115(4):983-90. doi: 10.1046/j.1365-2141.2001.03148.x. Br J Haematol. 2001. PMID: 11843837
-
Asparaginase (native ASNase or pegylated ASNase) in the treatment of acute lymphoblastic leukemia.Int J Nanomedicine. 2006;1(3):241-54. Int J Nanomedicine. 2006. PMID: 17717965 Free PMC article. Review.
-
Possible mechanism of metabolic and drug resistance with L-asparaginase therapy in childhood leukaemia.Front Oncol. 2023 Feb 1;13:1070069. doi: 10.3389/fonc.2023.1070069. eCollection 2023. Front Oncol. 2023. PMID: 36816964 Free PMC article. Review.
References
-
- Boos J. Pharmacokinetics and drug monitoring of L-Asparaginase treatment. Int J Clin Pharmacol Ther. (1997) 35:96–8. Epub 1997/03/01. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

