Elevated circulating monocytes and monocyte activation in COVID-19 convalescent individuals
- PMID: 37077911
- PMCID: PMC10106598
- DOI: 10.3389/fimmu.2023.1151780
Elevated circulating monocytes and monocyte activation in COVID-19 convalescent individuals
Abstract
Background: Monocytes and macrophages play a pivotal role in inflammation during acute SARS-CoV-2 infection. However, their contribution to the development of post-acute sequelae of SARS-CoV-2 infection (PASC) are not fully elucidated.
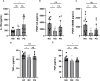
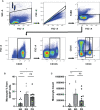
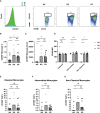
Methods: A cross-sectional study was conducted comparing plasma cytokine and monocyte levels among three groups: participants with pulmonary PASC (PPASC) with a reduced predicted diffusing capacity for carbon monoxide [DLCOc, <80%; (PG)]; fully recovered from SARS-CoV-2 with no residual symptoms (recovered group, RG); and negative for SARS-CoV-2 (negative group, NG). The expressions of cytokines were measured in plasma of study cohort by Luminex assay. The percentages and numbers of monocyte subsets (classical, intermediate, and non-classical monocytes) and monocyte activation (defined by CD169 expression) were analyzed using flow cytometry analysis of peripheral blood mononuclear cells.
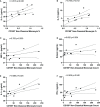
Results: Plasma IL-1Ra levels were elevated but FGF levels were reduced in PG compared to NG. Circulating monocytes and three subsets were significantly higher in PG and RG compared to NG. PG and RG exhibited higher levels of CD169+ monocyte counts and higher CD169 expression was detected in intermediate and non-classical monocytes from RG and PG than that found in NG. Further correlation analysis with CD169+ monocyte subsets revealed that CD169+ intermediate monocytes negatively correlated with DLCOc%, and CD169+ non-classical monocytes positively correlated with IL-1α, IL-1β, MIP-1α, Eotaxin, and IFN-γ.
Conclusion: This study present evidence that COVID convalescents exhibit monocyte alteration beyond the acute COVID-19 infection period even in convalescents with no residual symptoms. Further, the results suggest that monocyte alteration and increased activated monocyte subsets may impact pulmonary function in COVID-19 convalescents. This observation will aid in understanding the immunopathologic feature of pulmonary PASC development, resolution, and subsequent therapeutic interventions.
Keywords: CD169; Long-COVID; SARS-CoV-2; monocytes; post-acute sequalae of SARS-CoV-2 infection; pulmonary sequelae.
Copyright © 2023 Park, Dean, Jiyarom, Gangcuangco, Shah, Awamura, Ching, Nerurkar, Chow, Igno, Shikuma and Devendra.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

