A 6-month, Multi-center, Double-blind, Controlled Study to Evaluate the Effect of a Biofilm Disrupting Acne Cream on Mild-to-Moderate Facial Acne in Female Volunteer Subjects
- PMID: 37077927
- PMCID: PMC10110293
A 6-month, Multi-center, Double-blind, Controlled Study to Evaluate the Effect of a Biofilm Disrupting Acne Cream on Mild-to-Moderate Facial Acne in Female Volunteer Subjects
Abstract
Objectives: The primary aim of this study was to assess the change in acne lesions and severity within all treatment groups over the course of a six-month study.
Methods: This was a six-month, multisite, randomized, double-blind, controlled study in female subjects with mild-to-moderate acne to assess the clinical and psychological outcomes of treatment with biofilm disrupting acne cream 2x, biofilm disrupting acne cream 1x, biofilm disrupting acne cream without salicylic acid, 2.5% benzoyl peroxide (BPO) gel, and placebo. Subjects applied the assigned product to their face twice daily and were evaluated for clinical acne and quality of life outcomes at baseline and after six, 12, 18, and 24 weeks of treatment.
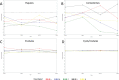
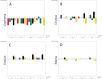
Results: After 24 weeks of use, subjects treated with biofilm disrupting acne cream 2x had a significantly greater improvement in the Investigator Global Assessment (IGA), compared to those treated with 2.5% BPO gel. Based on dermatologic assessments, biofilm disrupting acne cream 2x, biofilm disrupting acne cream 1x, biofilm disrupting acne cream without salicylic acid, and placebo control were associated with less erythema and dryness, compared to 2.5% BPO gel.
Limitations: Assessments within this study had the potential for subjective differences due to variability between evaluators.
Conclucion: Biofilm disrupting acne cream 2x and biofilm disrupting acne cream 1x provided equivalent efficacy to 2.5% BPO gel with less of the adverse effects commonly associated with BPO, such as erythema and dryness. Both the biofilm disrupting acne cream without salicylic acid and the placebo control were associated with mild improvements to acne symptoms over the course of the 24-week study.
Trial registry information: ClinicalTrials.gov, NCT03106766.
Keywords: Acne vulgaris; Cutibacterium acnes; Investigator’s Global Assessment; biofilm; lesions; quality of life; sebaceous gland disease; skin disease.
Copyright © 2023. Matrix Medical Communications. All rights reserved.
Conflict of interest statement
DISCLOSURES: Dr. Myntti and Ms. Porral and Palomo are employees of Next Science, LLC. All other authors report no conflicts of interest relevant to the contents of this article.
Figures


References
-
- Zaenglein AI, Pathy AI, Schlosser BJ et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945–973.e33. - PubMed
-
- Kircik LH. The role of benzoyl peroxide in the new treatment paradigm for acne. J Drugs Dermatol. 2013;12(6):s73–s76. - PubMed
-
- Ogé LK, Broussard A, Marshall MD. Acne vulgaris: diagnosis and treatment. Am Fam Physician. 2019;100(8):475–484. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
