Establishment and characterization of a multi-drug resistant cell line for canine mammary tumors
- PMID: 37077947
- PMCID: PMC10108679
- DOI: 10.3389/fvets.2023.1129756
Establishment and characterization of a multi-drug resistant cell line for canine mammary tumors
Abstract
Background and purpose: Canine mammary tumors are the most common tumor disease of female dogs, and adjuvant chemotherapy often results in multi-drug resistance. Currently, the mechanisms underlying the development of tumor multi-drug resistance are unclear. The translation of research applications that can be used to effectively overcome tumor resistance is similarly hampered. Therefore, it is urgent to construct multi-drug resistance models of canine mammary tumors that can be used for research, to explore the mechanisms and means of overcoming resistance.
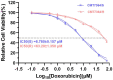
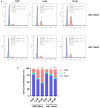
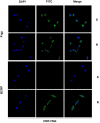
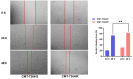
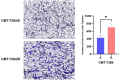
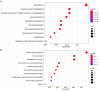
Materials and methods: In this study, the canine triple negative breast cancer cell line CMT-7364 was induced to develop multidrug resistance using doxorubicin by high-dose drug pulse method. The drug resistance and the expression of drug transport pumps of the cells was verified by CCK8 assay, immunoblotting, qPCR and immunofluorescence. Next, we used scratch assay and Transwell invasion assay to compare the migration and invasion abilities of the two cell lines and examined the expression of EMT-related proteins in both using immunoblotting. The differences of transcriptome between parental and drug-resistant cell lines were detected by RNA-seq sequencing. Finally, mouse xenograft models of drug-resistant and parental cell lines were constructed to evaluate the tumorigenic ability.
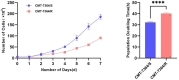
Results: After more than 50 generations of continuous passages stimulated by high-dose drug pulse method, the morphology of drug-resistant cell line CMT-7364/R tended to be mesenchymal-like and heterogeneous under light microscopy compared with the parental cell line CMT-7364/S, and developed resistance to doxorubicin and other commonly used chemotherapeutic drugs. In CMT-7364/R, BCRP was expressed at higher levels at both transcriptional and protein levels, while P-glycoprotein was not significantly different. Secondly, the migration and invasion ability of CMT-7364/R was significantly enhanced, with decreased expression of E-cadherin and increased expression of vimentin and mucin 1-N terminus. Finally, mouse xenograft models were constructed, while there was no significant difference in the volume of masses formed at 21 days.
Conclusion: In summary, by using the canine mammary tumor cell line CMT-7364/S as the parental cell line, we successfully constructed a multidrug-resistant CMT-7364/R with high-dose drug pulse methods. Compared to its parental cell line, CMT-7364/R has decreased growth rate, overexpression of BCRP and increased migration and invasion ability due to EMT. The results of this study showed that CMT-7364/R might serve as a model for future studies on tumor drug resistance.
Keywords: Breast Cancer Resistance Protein (BCRP or ABCG2); canine mammary tumor (CMT); chemotherapy; mRNA-sequencing (mRNA-seq); multidrug resistance (MDR).
Copyright © 2023 Zhou, Lin, Li, Zhang and Song.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Goldschmidt MH, Peña L, Zappulli V. Tumors of the mammary gland. In: Tumors in Domestic Animals. New Jersey: John Wiley & Sons, Inc. (2016). p. 723–65.
-
- Sorenmo KU, Worley DR, Zappulli V. Tumors of the mammary gland. In: Withrow and MacEwen's Small Animal Clinical Oncology. Amsterdam: (2019). p. 604–25.
LinkOut - more resources
Full Text Sources
Research Materials

