Extended physicochemical stability of cetuximab in opened vials and infusion bags when stored at 4°C and 25°C
- PMID: 37078110
- PMCID: PMC10804814
- DOI: 10.1177/10781552231170583
Extended physicochemical stability of cetuximab in opened vials and infusion bags when stored at 4°C and 25°C
Abstract
Introduction: This study aimed to determine the stability of cetuximab: (1) under "in-use" conditions after dilution to 1 mg/mL in 0.9% sodium chloride in polyolefin bags and (2) as an undiluted solution (5 mg/mL) repackaged in polypropylene bags or kept in the vial after opening.
Methods: Ready-to-use 500 mg/100 mL vials of cetuximab solution were diluted to 1 mg/mL in 100 mL bags of 0.9% sodium chloride or repackaged as a 5 mg/mL solution into empty 100 mL bags. Bags and vials were stored at 4°C for 90 days and 25°C for 3 days. A syringe sample of 7 mL was taken from each bag for the initial determinations. The sampled bags were weighed to determine their initial weight and placed under the planned storage conditions. The physicochemical stability of cetuximab was estimated using validated methods.
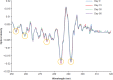
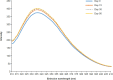
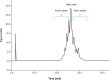
Results: No changes in turbidity, no protein loss, and no changes in cetuximab tertiary structure were observed after 30 days of storage or when subjected to a temperature excursion of 3 days at 25°C and when stored at 4°C for up to 90 days, regardless of the concentrations and batches. The colligative parameters did not change under any of the tested conditions. No evidence of microbial growth was found in bags after 90 days of storage at 4°C.
Conclusion: These results support the extended in-use shelf-life of cetuximab vials and bags, which can be cost-effective for healthcare providers.
Keywords: In-use stability; cetuximab; temperature excursion.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Similar articles
-
Long-term stability of morphine hydrochloride in 0.9% NaCl infusion polyolefin bags after freeze-thaw treatment and in polypropylene syringes at 5 degrees C + 3 degrees C.Int J Pharm Compd. 2014 Jan-Feb;18(1):78-82. Int J Pharm Compd. 2014. PMID: 24881344
-
Stability of cetuximab and panitumumab in glass vials and polyvinyl chloride bags.Am J Health Syst Pharm. 2010 Feb 1;67(3):223-6. doi: 10.2146/ajhp090031. Am J Health Syst Pharm. 2010. PMID: 20101065
-
Prolonged In-use Stability of Diluted Atezolizumab in Commercial Intravenous Bags.Int J Pharm Compd. 2021 May-Jun;25(3):246-257. Int J Pharm Compd. 2021. PMID: 34125716
-
Stability of doripenem in vitro in representative infusion solutions and infusion bags.Clin Ther. 2008 Nov;30(11):2075-87. doi: 10.1016/j.clinthera.2008.11.013. Clin Ther. 2008. PMID: 19108795
-
Stability of pemetrexed diarginine concentrates for solution in vials and diluted in 0.9% sodium chloride and dextrose 5% polyolefin infusion bags.Eur J Hosp Pharm. 2022 Nov;29(6):353-358. doi: 10.1136/ejhpharm-2020-002620. Epub 2021 Mar 3. Eur J Hosp Pharm. 2022. PMID: 33658227 Free PMC article.
Cited by
-
Integrating Analytical Procedures in Routine Practices of Centralized Antiblastic Compounding Units for Valorization of Residual Compounded Drugs.Pharmaceutics. 2025 Jan 14;17(1):101. doi: 10.3390/pharmaceutics17010101. Pharmaceutics. 2025. PMID: 39861749 Free PMC article.
References
-
- Erbitux® (package insert). Indianapolis, IN: Elly Lilly and Company, 2021.
-
- Erbitux® (package insert). Darmstadt, Germany: Merck KGaA, 2020.
-
- Council of Europe. Monoclonal antibodies for human use. In: European Pharmacopoeia (Ph. Eur.) 10th ed. Strasbourg: 2021, vol. 01/2012.
-
- Bardo-Brouard P, Vieillard V, Shekarian T, et al. Stability of ipilimumab in its original vial after opening allows its use for at least 4 weeks and facilitates pooling of residues. Eur J Cancer 2016; 58: 8–16. - PubMed
-
- Farjami A, Siahi-Shadbad M, Akbarzadehlaleh P, et al. Evaluation of the physicochemical and biological stability of cetuximab under various stress condition. J Pharm Pharm Sci 2019; 22: 171–190. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

