Prognostic Models for Global Functional Outcome and Post-Concussion Symptoms Following Mild Traumatic Brain Injury: A Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) Study
- PMID: 37078144
- PMCID: PMC10458380
- DOI: 10.1089/neu.2022.0320
Prognostic Models for Global Functional Outcome and Post-Concussion Symptoms Following Mild Traumatic Brain Injury: A Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) Study
Abstract
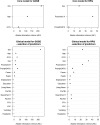
After mild traumatic brain injury (mTBI), a substantial proportion of individuals do not fully recover on the Glasgow Outcome Scale Extended (GOSE) or experience persistent post-concussion symptoms (PPCS). We aimed to develop prognostic models for the GOSE and PPCS at 6 months after mTBI and to assess the prognostic value of different categories of predictors (clinical variables; questionnaires; computed tomography [CT]; blood biomarkers). From the Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) study, we included participants aged 16 or older with Glasgow Coma Score (GCS) 13-15. We used ordinal logistic regression to model the relationship between predictors and the GOSE, and linear regression to model the relationship between predictors and the Rivermead Post-concussion Symptoms Questionnaire (RPQ) total score. First, we studied a pre-specified Core model. Next, we extended the Core model with other clinical and sociodemographic variables available at presentation (Clinical model). The Clinical model was then extended with variables assessed before discharge from hospital: early post-concussion symptoms, CT variables, biomarkers, or all three categories (extended models). In a subset of patients mostly discharged home from the emergency department, the Clinical model was extended with 2-3-week post-concussion and mental health symptoms. Predictors were selected based on Akaike's Information Criterion. Performance of ordinal models was expressed as a concordance index (C) and performance of linear models as proportion of variance explained (R2). Bootstrap validation was used to correct for optimism. We included 2376 mTBI patients with 6-month GOSE and 1605 patients with 6-month RPQ. The Core and Clinical models for GOSE showed moderate discrimination (C = 0.68 95% confidence interval 0.68 to 0.70 and C = 0.70[0.69 to 0.71], respectively) and injury severity was the strongest predictor. The extended models had better discriminative ability (C = 0.71[0.69 to 0.72] with early symptoms; 0.71[0.70 to 0.72] with CT variables or with blood biomarkers; 0.72[0.71 to 0.73] with all three categories). The performance of models for RPQ was modest (R2 = 4% Core; R2 = 9% Clinical), and extensions with early symptoms increased the R2 to 12%. The 2-3-week models had better performance for both outcomes in the subset of participants with these symptoms measured (C = 0.74 [0.71 to 0.78] vs. C = 0.63[0.61 to 0.67] for GOSE; R2 = 37% vs. 6% for RPQ). In conclusion, the models based on variables available before discharge have moderate performance for the prediction of GOSE and poor performance for the prediction of PPCS. Symptoms assessed at 2-3 weeks are required for better predictive ability of both outcomes. The performance of the proposed models should be examined in independent cohorts.
Keywords: Glasgow Outcome Scale Extended; biomarkers; mild traumatic brain injury; post-concussion symptoms; predictors; prognostic model.
Conflict of interest statement
DM received personal fees from Lantmannen AB, GlaxoSmithKline plc, Calico Life Sciences LLC, PresSura Neuro, Integra Neurosciences, and NeuroTrauma Sciences, LLC; grants from GlaxoSmithKline plc; and a shared National Institutes of Health grant from Gryphon Collaborators on a grant application outside the presented work. VFJN holds grants from Roche Pharmaceuticals for an analysis outside the presented work. AIRM declares personal fees from NeuroTrauma Sciences and Novartis and participated on the DSMB of PresSura Neuro during the conduct of the study.
For the other authors, no competing financial interests exist.
Figures



Similar articles
-
Prediction of Global Functional Outcome and Post-Concussive Symptoms after Mild Traumatic Brain Injury: External Validation of Prognostic Models in the Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) Study.J Neurotrauma. 2021 Jan 15;38(2):196-209. doi: 10.1089/neu.2020.7074. Epub 2020 Oct 19. J Neurotrauma. 2021. PMID: 32977737
-
Pathological Computed Tomography Features Associated With Adverse Outcomes After Mild Traumatic Brain Injury: A TRACK-TBI Study With External Validation in CENTER-TBI.JAMA Neurol. 2021 Sep 1;78(9):1137-1148. doi: 10.1001/jamaneurol.2021.2120. JAMA Neurol. 2021. PMID: 34279565 Free PMC article.
-
Early predictors of outcome after mild traumatic brain injury (UPFRONT): an observational cohort study.Lancet Neurol. 2017 Jul;16(7):532-540. doi: 10.1016/S1474-4422(17)30117-5. Epub 2017 Jun 13. Lancet Neurol. 2017. PMID: 28653646
-
Exploring Serum Biomarkers for Mild Traumatic Brain Injury.In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 22. In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 22. PMID: 26269900 Free Books & Documents. Review.
-
Animal Models for Concussion: Molecular and Cognitive Assessments—Relevance to Sport and Military Concussions.In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 46. In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 46. PMID: 26269898 Free Books & Documents. Review.
Cited by
-
mTBI Biological Biomarkers as Predictors of Postconcussion Syndrome-Review.Brain Sci. 2024 May 18;14(5):513. doi: 10.3390/brainsci14050513. Brain Sci. 2024. PMID: 38790491 Free PMC article. Review.
-
Inhibition of mannan-binding lectin associated serine protease (MASP)-2 reduces the cognitive deficits in a mouse model of severe traumatic brain injury.J Neuroinflammation. 2024 May 28;21(1):141. doi: 10.1186/s12974-024-03133-4. J Neuroinflammation. 2024. PMID: 38807149 Free PMC article.
-
Predicting recovery in patients with mild traumatic brain injury and a normal CT using serum biomarkers and diffusion tensor imaging (CENTER-TBI): an observational cohort study.EClinicalMedicine. 2024 Aug 8;75:102751. doi: 10.1016/j.eclinm.2024.102751. eCollection 2024 Sep. EClinicalMedicine. 2024. PMID: 39720677 Free PMC article.
-
Examining and comparing the clinical characteristics of adults with persisting post-concussion symptoms presenting for outpatient rehabilitation following a mild traumatic brain injury or a minimal head injury.J Rehabil Med. 2025 Jul 1;57:jrm43506. doi: 10.2340/jrm.v57.43506. J Rehabil Med. 2025. PMID: 40598804 Free PMC article.
-
Maximizing the Clinical Value of Blood-Based Biomarkers for Mild Traumatic Brain Injury.Diagnostics (Basel). 2023 Oct 28;13(21):3330. doi: 10.3390/diagnostics13213330. Diagnostics (Basel). 2023. PMID: 37958226 Free PMC article. Review.
References
-
- Nelson LD, Temkin NR, Dikmen S, et al. Recovery after mild traumatic brain injury in patients presenting to US level I trauma centers: a Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) study. JAMA Neurol 2019;76(9):1049–1059; doi: 10.1001/jamaneurol.2019.1313 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
