New insights into the pathophysiology of methylmalonic acidemia
- PMID: 37078237
- PMCID: PMC10715492
- DOI: 10.1002/jimd.12617
New insights into the pathophysiology of methylmalonic acidemia
Abstract
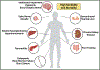
Methylmalonic acidemia (MMA) is a severe inborn error of metabolism that is characterized by pleiotropic metabolic perturbations and multiorgan pathology. Treatment options are limited and non-curative as the underlying causative molecular mechanisms remain unknown. While earlier studies have focused on the potential direct toxicity of metabolites such as methylmalonic and propionic acid as a mechanism to explain disease pathophysiology, new observations have revealed that aberrant acylation, specifically methylmalonylation, is a characteristic feature of MMA. The mitochondrial sirtuin enzyme SIRT5 is capable of recognizing and removing this PTM, however, reduced protein levels of SIRT5 along with other mitochondrial SIRTs 3 and 4 in MMA and potentially reduced function of all three indicates aberrant acylation may require clinical intervention. Therefore, targeting posttranslational modifications may represent a new therapeutic approach to treat MMA and related organic acidemias.
Keywords: MMA; PTM; methylmalonyl-CoA mutase; organic acidemia; sirtuin.
Published 2023. This article is a U.S. Government work and is in the public domain in the USA.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
Venditti reports previous nonfinancial support and other from Moderna Therapeutics, nonfinancial support and other from LogicBio Therapeutics, nonfinancial support and other from Translate Bio, nonfinancial support and other from AskBio, all outside the submitted work. Venditti currently receives nonfinancial support and other from Selecta Biosciences. In addition, on the behalf of Venditti and Head, the NIH has filed of number of patents related to medical devices, gene therapy, and biomarkers. Meier reports no conflicts.
Figures



Similar articles
-
Aberrant methylmalonylation underlies methylmalonic acidemia and is attenuated by an engineered sirtuin.Sci Transl Med. 2022 May 25;14(646):eabn4772. doi: 10.1126/scitranslmed.abn4772. Epub 2022 May 25. Sci Transl Med. 2022. PMID: 35613279 Free PMC article.
-
Biochemical and anaplerotic applications of in vitro models of propionic acidemia and methylmalonic acidemia using patient-derived primary hepatocytes.Mol Genet Metab. 2020 Jul;130(3):183-196. doi: 10.1016/j.ymgme.2020.05.003. Epub 2020 May 11. Mol Genet Metab. 2020. PMID: 32451238 Free PMC article.
-
A novel small molecule approach for the treatment of propionic and methylmalonic acidemias.Mol Genet Metab. 2021 May;133(1):71-82. doi: 10.1016/j.ymgme.2021.03.001. Epub 2021 Mar 10. Mol Genet Metab. 2021. PMID: 33741272 Free PMC article.
-
Treatment of metabolic disorders using genomic technologies: Lessons from methylmalonic acidemia.J Inherit Metab Dis. 2022 Sep;45(5):872-888. doi: 10.1002/jimd.12534. Epub 2022 Jul 21. J Inherit Metab Dis. 2022. PMID: 35766386 Review.
-
Biomarkers to predict disease progression and therapeutic response in isolated methylmalonic acidemia.J Inherit Metab Dis. 2023 Jul;46(4):554-572. doi: 10.1002/jimd.12636. Epub 2023 Jun 6. J Inherit Metab Dis. 2023. PMID: 37243446 Free PMC article. Review.
Cited by
-
Improving the second-tier classification of methylmalonic acidemia patients using a machine learning ensemble method.World J Pediatr. 2024 Oct;20(10):1090-1101. doi: 10.1007/s12519-023-00788-6. Epub 2024 Feb 24. World J Pediatr. 2024. PMID: 38401044 Free PMC article.
-
The Arg108Cys Variant of Methylmalonyl-CoA Mutase: Clinical Implications for the Mexican Population Based on Molecular Dynamics and Docking.Int J Mol Sci. 2025 Mar 22;26(7):2887. doi: 10.3390/ijms26072887. Int J Mol Sci. 2025. PMID: 40243530 Free PMC article.
-
Isolated methylmalonic acidemia in Mexico: Genotypic spectrum, report of two novel MMUT variants and a possible synergistic heterozygosity effect.Mol Genet Metab Rep. 2024 Oct 16;41:101155. doi: 10.1016/j.ymgmr.2024.101155. eCollection 2024 Dec. Mol Genet Metab Rep. 2024. PMID: 39494389 Free PMC article.
-
Successful Pregnancy Management of a Woman With Severe Methylmalonic Acidemia.JIMD Rep. 2025 May 12;66(3):e70009. doi: 10.1002/jmd2.70009. eCollection 2025 May. JIMD Rep. 2025. PMID: 40365323 Free PMC article.
-
An Explorative Qualitative Study of the Role of a Genetic Counsellor to Parents Receiving a Diagnosis After a Positive Newborn Bloodspot Screening.Int J Neonatal Screen. 2025 Apr 28;11(2):32. doi: 10.3390/ijns11020032. Int J Neonatal Screen. 2025. PMID: 40407515 Free PMC article.
References
-
- Stokke O, Eldjarn L, Norum KR, Steenjoh J, Halvorsen S. Methylmalonic acidemia—a new inborn error of metabolism which may cause fatal acidosis in neonatal period. Scand J Clin Lab Inv 1967;20:313–328.
-
- Rosenberg LE, Lilljeqvist A, Hsia YE. Methylmalonic aciduria: metabolic block localization and vitamin B 12 dependency. Science 1968;162:805–807. - PubMed
-
- Rosenberg LE, Lilljeqvist AC, Hsia YE. Methylmalonic aciduria—an inborn error leading to metabolic acidosis, long-chain ketonuria and intermittent hyperglycinemia. N Engl J Med 1968;278:1319–1322. - PubMed
-
- Childs B, Nyhan WL, Borden M, Bard L, Cooke RE. Idiopathic hyperglycinemia and hyperglycinuria: a new disorder of amino acid metabolism. I Pediatrics 1961;27:522–538. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

