Magnetic Resonance Imaging of Mouse Cerebral Cavernomas Reveal Differential Lesion Progression and Variable Permeability to Gadolinium
- PMID: 37078284
- PMCID: PMC10257814
- DOI: 10.1161/ATVBAHA.122.318938
Magnetic Resonance Imaging of Mouse Cerebral Cavernomas Reveal Differential Lesion Progression and Variable Permeability to Gadolinium
Abstract
Background: Cerebral cavernous malformations, also known as cavernous angiomas, are blood vessel abnormalities comprised of clusters of grossly enlarged and hemorrhage-prone capillaries. The prevalence in the general population, including asymptomatic cases, is estimated to be 0.5%. Some patients develop severe symptoms, including seizures and focal neurological deficits, whereas others remain asymptomatic. The causes of this remarkable presentation heterogeneity within a primarily monogenic disease remain poorly understood.
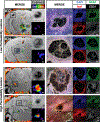
Methods: We established a chronic mouse model of cerebral cavernous malformations, induced by postnatal ablation of Krit1 with Pdgfb-CreERT2, and examined lesion progression in these mice with T2-weighted 7T magnetic resonance imaging (MRI). We also established a modified protocol for dynamic contrast-enhanced MRI and produced quantitative maps of gadolinium tracer gadobenate dimeglumine. After terminal imaging, brain slices were stained with antibodies against microglia, astrocytes, and endothelial cells.
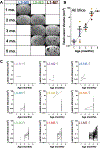
Results: These mice develop cerebral cavernous malformations lesions gradually over 4 to 5 months of age throughout the brain. Precise volumetric analysis of individual lesions revealed nonmonotonous behavior, with some lesions temporarily growing smaller. However, the cumulative lesional volume invariably increased over time and after about 2 months followed a power trend. Using dynamic contrast-enhanced MRI, we produced quantitative maps of gadolinium in the lesions, indicating a high degree of heterogeneity in lesional permeability. MRI properties of the lesions were correlated with cellular markers for endothelial cells, astrocytes, and microglia. Multivariate comparisons of MRI properties of the lesions with cellular markers for endothelial and glial cells revealed that increased cell density surrounding lesions correlates with stability, whereas denser vasculature within and surrounding the lesions may correlate with high permeability.
Conclusions: Our results lay a foundation for better understanding individual lesion properties and provide a comprehensive preclinical platform for testing new drug and gene therapies for controlling cerebral cavernous malformations.
Keywords: astrocytes; endothelial cells; gadolinium; hemorrhage; magnetic resonance imaging.
Conflict of interest statement
Figures


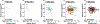
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

