Lineage-Specific Induced Pluripotent Stem Cell-Derived Smooth Muscle Cell Modeling Predicts Integrin Alpha-V Antagonism Reduces Aortic Root Aneurysm Formation in Marfan Syndrome Mice
- PMID: 37078287
- PMCID: PMC10330156
- DOI: 10.1161/ATVBAHA.122.318448
Lineage-Specific Induced Pluripotent Stem Cell-Derived Smooth Muscle Cell Modeling Predicts Integrin Alpha-V Antagonism Reduces Aortic Root Aneurysm Formation in Marfan Syndrome Mice
Abstract
Background: The role of increased smooth muscle cell (SMC) integrin αv signaling in Marfan syndrome (MFS) aortic aneurysm remains unclear. Herein, we examine the mechanism and potential efficacy of integrin αv blockade as a therapeutic strategy to reduce aneurysm progression in MFS.
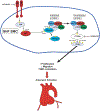
Methods: Induced pluripotent stem cells (iPSCs) were differentiated into aortic SMCs of the second heart field (SHF) and neural crest (NC) lineages, enabling in vitro modeling of MFS thoracic aortic aneurysms. The pathological role of integrin αv during aneurysm formation was confirmed by blockade of integrin αv with GLPG0187 in Fbn1C1039G/+ MFS mice.
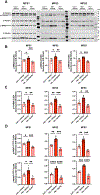
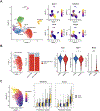
Results: iPSC-derived MFS SHF SMCs overexpress integrin αv relative to MFS NC and healthy control SHF cells. Furthermore, integrin αv downstream targets (FAK [focal adhesion kinase]/AktThr308/mTORC1 [mechanistic target of rapamycin complex 1]) were activated, especially in MFS SHF. Treatment of MFS SHF SMCs with GLPG0187 reduced p-FAK/p-AktThr308/mTORC1 activity back to control SHF levels. Functionally, MFS SHF SMCs had increased proliferation and migration compared to MFS NC SMCs and control SMCs, which normalized with GLPG0187 treatment. In the Fbn1C1039G/+ MFS mouse model, integrin αv, p-AktThr308, and downstream targets of mTORC1 proteins were elevated in the aortic root/ascending segment compared to littermate wild-type control. Mice treated with GLPG0187 (age 6-14 weeks) had reduced aneurysm growth, elastin fragmentation, and reduction of the FAK/AktThr308/mTORC1 pathway. GLPG0187 treatment reduced the amount and severity of SMC modulation assessed by single-cell RNA sequencing.
Conclusions: The integrin αv-FAK-AktThr308 signaling pathway is activated in iPSC SMCs from MFS patients, specifically from the SHF lineage. Mechanistically, this signaling pathway promotes SMC proliferation and migration in vitro. As biological proof of concept, GLPG0187 treatment slowed aneurysm growth and p-AktThr308 signaling in Fbn1C1039G/+ mice. Integrin αv blockade via GLPG0187 may be a promising therapeutic approach to inhibit MFS aneurysmal growth.
Keywords: Marfan syndrome; aortic aneurysm; extracellular matrix; phenotype; proteomic; transcriptome; vascular remodeling.
Conflict of interest statement
Figures







Comment in
-
Altered Integrin Signaling in Thoracic Aortopathy.Arterioscler Thromb Vasc Biol. 2023 Jul;43(7):1154-1156. doi: 10.1161/ATVBAHA.123.319404. Epub 2023 May 11. Arterioscler Thromb Vasc Biol. 2023. PMID: 37165879 No abstract available.
Similar articles
-
Embryologic Origin Influences Smooth Muscle Cell Phenotypic Modulation Signatures in Murine Marfan Syndrome Aortic Aneurysm.Arterioscler Thromb Vasc Biol. 2022 Sep;42(9):1154-1168. doi: 10.1161/ATVBAHA.122.317381. Epub 2022 Jul 7. Arterioscler Thromb Vasc Biol. 2022. PMID: 35861960 Free PMC article.
-
Quantitative proteomics reveal lineage-specific protein profiles in iPSC-derived Marfan syndrome smooth muscle cells.Sci Rep. 2020 Nov 23;10(1):20392. doi: 10.1038/s41598-020-77274-w. Sci Rep. 2020. PMID: 33230159 Free PMC article.
-
Single-Cell Transcriptomic Profiling of Vascular Smooth Muscle Cell Phenotype Modulation in Marfan Syndrome Aortic Aneurysm.Arterioscler Thromb Vasc Biol. 2020 Sep;40(9):2195-2211. doi: 10.1161/ATVBAHA.120.314670. Epub 2020 Jul 23. Arterioscler Thromb Vasc Biol. 2020. PMID: 32698686 Free PMC article.
-
Insights into elastic fiber fragmentation: Mechanisms and treatment of aortic aneurysm in Marfan syndrome.Vascul Pharmacol. 2023 Dec;153:107215. doi: 10.1016/j.vph.2023.107215. Epub 2023 Aug 26. Vascul Pharmacol. 2023. PMID: 37640090 Free PMC article. Review.
-
Molecular pathogenesis of Marfan syndrome.Int J Cardiol. 2015;187:585-91. doi: 10.1016/j.ijcard.2015.03.423. Epub 2015 Mar 31. Int J Cardiol. 2015. PMID: 25863307 Review.
Cited by
-
Transcriptional regulation of postnatal aortic development.Cells Dev. 2024 Dec;180:203971. doi: 10.1016/j.cdev.2024.203971. Epub 2024 Oct 18. Cells Dev. 2024. PMID: 39426523
-
Therapeutic Opportunities of Marfan Syndrome: Current Perspectives.Drug Des Devel Ther. 2025 Aug 26;19:7365-7379. doi: 10.2147/DDDT.S523571. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40901270 Free PMC article. Review.
-
Integrin-mediated mTOR signaling drives TGF-β overactivity and myxomatous mitral valve degeneration in hypomorphic fibrillin-1 mice.J Clin Invest. 2025 May 20;135(14):e183558. doi: 10.1172/JCI183558. eCollection 2025 Jul 15. J Clin Invest. 2025. PMID: 40392604 Free PMC article.
-
Single-Cell Transcriptomics Identifies Selective Lineage-Specific Regulation of Genes in Aortic Smooth Muscle Cells in Mice.Arterioscler Thromb Vasc Biol. 2025 Feb;45(2):e15-e29. doi: 10.1161/ATVBAHA.124.321482. Epub 2025 Jan 2. Arterioscler Thromb Vasc Biol. 2025. PMID: 39744838
-
Use of iPSC-Derived Smooth Muscle Cells to Model Physiology and Pathology.Arterioscler Thromb Vasc Biol. 2024 Jul;44(7):1523-1536. doi: 10.1161/ATVBAHA.123.319703. Epub 2024 May 2. Arterioscler Thromb Vasc Biol. 2024. PMID: 38695171 Free PMC article. Review.
References
-
- Dietz HC, Cutting CR, Pyeritz RE, Maslen CL, Sakai LY, Corson GM, Puffenberger EG, Hamosh A, Nanthakumar EJ, Curristin SM, Stetten G, Meyers DA, Francomano CA. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352(6333):337–339. doi:10.1038/352337a0 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

