Photocleavable Ortho-Nitrobenzyl-Protected DNA Architectures and Their Applications
- PMID: 37078690
- PMCID: PMC10214457
- DOI: 10.1021/acs.chemrev.3c00016
Photocleavable Ortho-Nitrobenzyl-Protected DNA Architectures and Their Applications
Abstract
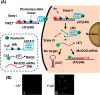
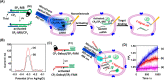
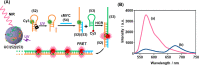
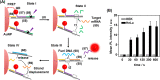
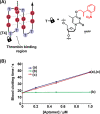
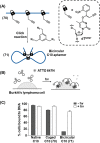
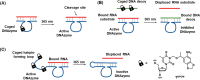
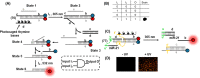
This review article introduces mechanistic aspects and applications of photochemically deprotected ortho-nitrobenzyl (ONB)-functionalized nucleic acids and their impact on diverse research fields including DNA nanotechnology and materials chemistry, biological chemistry, and systems chemistry. Specific topics addressed include the synthesis of the ONB-modified nucleic acids, the mechanisms involved in the photochemical deprotection of the ONB units, and the photophysical and chemical means to tune the irradiation wavelength required for the photodeprotection process. Principles to activate ONB-caged nanostructures, ONB-protected DNAzymes and aptamer frameworks are introduced. Specifically, the use of ONB-protected nucleic acids for the phototriggered spatiotemporal amplified sensing and imaging of intracellular mRNAs at the single-cell level are addressed, and control over transcription machineries, protein translation and spatiotemporal silencing of gene expression by ONB-deprotected nucleic acids are demonstrated. In addition, photodeprotection of ONB-modified nucleic acids finds important applications in controlling material properties and functions. These are introduced by the phototriggered fusion of ONB nucleic acid functionalized liposomes as models for cell-cell fusion, the light-stimulated fusion of ONB nucleic acid functionalized drug-loaded liposomes with cells for therapeutic applications, and the photolithographic patterning of ONB nucleic acid-modified interfaces. Particularly, the photolithographic control of the stiffness of membrane-like interfaces for the guided patterned growth of cells is realized. Moreover, ONB-functionalized microcapsules act as light-responsive carriers for the controlled release of drugs, and ONB-modified DNA origami frameworks act as mechanical devices or stimuli-responsive containments for the operation of DNA machineries such as the CRISPR-Cas9 system. The future challenges and potential applications of photoprotected DNA structures are discussed.
Conflict of interest statement
The authors declare no competing financial interest.
Figures











Similar articles
-
Dynamic Fusion of Nucleic Acid Functionalized Nano-/Micro-Cell-Like Containments: From Basic Concepts to Applications.ACS Nano. 2023 Aug 22;17(16):15308-15327. doi: 10.1021/acsnano.3c04415. Epub 2023 Aug 7. ACS Nano. 2023. PMID: 37549398 Free PMC article. Review.
-
Aptamer-Functionalized Hybrid Nanostructures for Sensing, Drug Delivery, Catalysis and Mechanical Applications.Int J Mol Sci. 2021 Feb 11;22(4):1803. doi: 10.3390/ijms22041803. Int J Mol Sci. 2021. PMID: 33670386 Free PMC article. Review.
-
Near-infrared light-activated membrane fusion for cancer cell therapeutic applications.Chem Sci. 2020 May 7;11(21):5592-5600. doi: 10.1039/d0sc00863j. eCollection 2020 Jun 7. Chem Sci. 2020. PMID: 32874503 Free PMC article.
-
Stimuli-Responsive DNA-Based Hydrogels: From Basic Principles to Applications.Acc Chem Res. 2017 Apr 18;50(4):680-690. doi: 10.1021/acs.accounts.6b00542. Epub 2017 Mar 1. Acc Chem Res. 2017. PMID: 28248486
-
Scaffolding along nucleic acid duplexes using 2'-amino-locked nucleic acids.Acc Chem Res. 2014 Jun 17;47(6):1768-77. doi: 10.1021/ar500014g. Epub 2014 Apr 21. Acc Chem Res. 2014. PMID: 24749544
Cited by
-
Functional Nucleic Acid Probes Based on Two-Photon for Biosensing.Biosensors (Basel). 2023 Aug 23;13(9):836. doi: 10.3390/bios13090836. Biosensors (Basel). 2023. PMID: 37754070 Free PMC article. Review.
-
Spatiotemporal dynamic and catalytically mediated reconfiguration of compartmentalized cyanuric acid/polyadenine DNA microdroplet condensates.Nat Commun. 2025 Apr 9;16(1):3352. doi: 10.1038/s41467-025-58650-4. Nat Commun. 2025. PMID: 40204808 Free PMC article.
-
Photochemically Triggered, Transient, and Oscillatory Transcription Machineries Guide Temporal Modulation of Fibrinogenesis.J Am Chem Soc. 2025 Jan 15;147(2):2216-2227. doi: 10.1021/jacs.4c16829. Epub 2024 Dec 31. J Am Chem Soc. 2025. PMID: 39740143 Free PMC article.
-
On-Demand Photoactivation of DNA-Based Motor Motion.ACS Nano. 2025 Feb 11;19(5):5363-5375. doi: 10.1021/acsnano.4c13068. Epub 2025 Jan 30. ACS Nano. 2025. PMID: 39883883 Free PMC article.
-
Temporal logic circuits implementation using a dual cross-inhibition mechanism based on DNA strand displacement.RSC Adv. 2023 Sep 11;13(39):27125-27134. doi: 10.1039/d3ra03995a. eCollection 2023 Sep 8. RSC Adv. 2023. PMID: 37701285 Free PMC article.
References
-
- Merrifield R. B. Solid Phase Peptide Synthesis. I. The Synthesis of a Tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. 10.1021/ja00897a025. - DOI
-
- Hartrampf N.; Saebi A.; Poskus M.; Gates Z. P.; Callahan A. J.; Cowfer A. E.; Hanna S.; Antilla S.; Schissel C. K.; Quartararo A. J.; Ye X.; Mijalis A. J.; Simon M. D.; Loas A.; Liu S.; Jessen C.; Nielsen T. E.; Pentelute B. L. Synthesis of Proteins by Automated Flow Chemistry. Science 2020, 368, 980–987. 10.1126/science.abb2491. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

