Shear and hydrostatic stress regulate fetal heart valve remodeling through YAP-mediated mechanotransduction
- PMID: 37078699
- PMCID: PMC10162797
- DOI: 10.7554/eLife.83209
Shear and hydrostatic stress regulate fetal heart valve remodeling through YAP-mediated mechanotransduction
Abstract
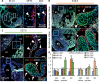
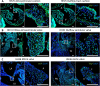
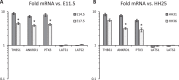
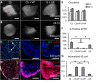
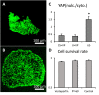
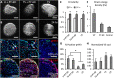
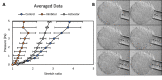
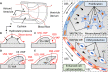
Clinically serious congenital heart valve defects arise from improper growth and remodeling of endocardial cushions into leaflets. Genetic mutations have been extensively studied but explain less than 20% of cases. Mechanical forces generated by beating hearts drive valve development, but how these forces collectively determine valve growth and remodeling remains incompletely understood. Here, we decouple the influence of those forces on valve size and shape, and study the role of YAP pathway in determining the size and shape. The low oscillatory shear stress promotes YAP nuclear translocation in valvular endothelial cells (VEC), while the high unidirectional shear stress restricts YAP in cytoplasm. The hydrostatic compressive stress activated YAP in valvular interstitial cells (VIC), whereas the tensile stress deactivated YAP. YAP activation by small molecules promoted VIC proliferation and increased valve size. Whereas YAP inhibition enhanced the expression of cell-cell adhesions in VEC and affected valve shape. Finally, left atrial ligation was performed in chick embryonic hearts to manipulate the shear and hydrostatic stress in vivo. The restricted flow in the left ventricle induced a globular and hypoplastic left atrioventricular (AV) valves with an inhibited YAP expression. By contrast, the right AV valves with sustained YAP expression grew and elongated normally. This study establishes a simple yet elegant mechanobiological system by which transduction of local stresses regulates valve growth and remodeling. This system guides leaflets to grow into proper sizes and shapes with the ventricular development, without the need of a genetically prescribed timing mechanism.
Keywords: biomechanics; chicken; congenital heart defect; developmental biology; maturation; mechanobiology; morphogenesis; mouse.
© 2023, Wang et al.
Conflict of interest statement
MW, BL, SS, CD, FL, JB No competing interests declared
Figures


Update of
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

