Early myelination involves the dynamic and repetitive ensheathment of axons which resolves through a low and consistent stabilization rate
- PMID: 37078701
- PMCID: PMC10198724
- DOI: 10.7554/eLife.82111
Early myelination involves the dynamic and repetitive ensheathment of axons which resolves through a low and consistent stabilization rate
Abstract
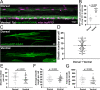
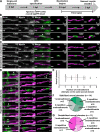
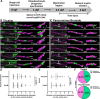
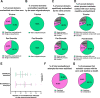
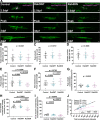
Oligodendrocytes in the central nervous system exhibit significant variability in the number of myelin sheaths that are supported by each cell, ranging from 1 to 50 (1-8). Myelin production during development is dynamic and involves both sheath formation and loss (3, 9-13). However, how these parameters are balanced to generate this heterogeneity in sheath number has not been thoroughly investigated. To explore this question, we combined extensive time-lapse and longitudinal imaging of oligodendrocytes in the developing zebrafish spinal cord to quantify sheath initiation and loss. Surprisingly, we found that oligodendrocytes repetitively ensheathed the same axons multiple times before any stable sheaths were formed. Importantly, this repetitive ensheathment was independent of neuronal activity. At the level of individual oligodendrocytes, each cell initiated a highly variable number of total ensheathments. However, ~80-90% of these ensheathments always disappeared, an unexpectedly high, but consistent rate of loss. The dynamics of this process indicated rapid membrane turnover as ensheathments were formed and lost repetitively on each axon. To better understand how these sheath initiation dynamics contribute to sheath accumulation and stabilization, we disrupted membrane recycling by expressing a dominant-negative mutant form of Rab5. Oligodendrocytes over-expressing this mutant did not show a change in early sheath initiation dynamics but did lose a higher percentage of ensheathments in the later stabilization phase. Overall, oligodendrocyte sheath number is heterogeneous because each cell repetitively initiates a variable number of total ensheathments that are resolved through a consistent stabilization rate.
Keywords: endocytic recycling; ensheathment dynamics; myelination; neuroscience; oligodendrocytes; zebrafish.
© 2023, Almeida and Macklin.
Conflict of interest statement
AA, WM No competing interests declared
Figures








Update of
- doi: 10.1101/2022.07.17.500324
References
-
- Baron W, Ozgen H, Klunder B, de Jonge JC, Nomden A, Plat A, Trifilieff E, de Vries H, Hoekstra D. The major myelin-resident protein PLP is transported to myelin membranes via a transcytotic mechanism: involvement of sulfatide. Molecular and Cellular Biology. 2015;35:288–302. doi: 10.1128/MCB.00848-14. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

