Enzyme-Triggered Chemodynamic Therapy via a Peptide-H2 S Donor Conjugate with Complexed Fe2
- PMID: 37078735
- PMCID: PMC10241505
- DOI: 10.1002/anie.202302303
Enzyme-Triggered Chemodynamic Therapy via a Peptide-H2 S Donor Conjugate with Complexed Fe2
Abstract
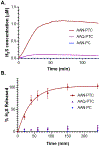
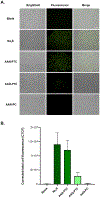
Inducing high levels of reactive oxygen species (ROS) inside tumor cells is a cancer therapy method termed chemodynamic therapy (CDT). Relying on delivery of Fenton reaction promoters such as Fe2+ , CDT takes advantage of overproduced ROS in the tumor microenvironment. We developed a peptide-H2 S donor conjugate, complexed with Fe2+ , termed AAN-PTC-Fe2+ . The AAN tripeptide was specifically cleaved by legumain, an enzyme overexpressed in glioma cells, to release carbonyl sulfide (COS). Hydrolysis of COS by carbonic anhydrase formed H2 S, an inhibitor of catalase, an enzyme that detoxifies H2 O2 . Fe2+ and H2 S together increased intracellular ROS levels and decreased viability in C6 glioma cells compared with controls lacking either Fe2+ , the AAN sequence, or the ability to generate H2 S. AAN-PTC-Fe2+ performed better than temezolimide while exhibiting no cytotoxicity toward H9C2 cardiomyocytes. This study provides an H2 S-amplified, enzyme-responsive platform for synergistic cancer treatment.
Keywords: Cancer; Drug Delivery; Gasotransmitter; Iron; Prodrugs.
© 2023 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Figures
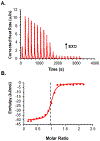


References
-
- Wang J, Yi J, Cancer Biol. Ther 2008, 7, 1875–1884. - PubMed
-
- Zhang C, Bu W, Ni D, Zhang S, Li Q, Yao Z, Zhang J, Yao H, Wang Z, Shi J, Angew. Chemie Int. Ed 2016, 55, 2101–2106. - PubMed
-
- Li X, Zhou Q, Japir AA-WMM, Dutta D, Lu N, Ge Z, ACS Nano 2022, 16, 14982–14999. - PubMed
-
- Zhao P, Li H, Bu W, Angew. Chemie Int. Ed 2023, 62, e202210415. - PubMed
-
- Wang X, Zhong X, Liu Z, Cheng L, Nano Today 2020, 35, 100946.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

